Researchers noticed 3-phenyllactic acid in the early 1900s, tucked away in metabolic studies of bacteria and fungi. Early biochemists worked to isolate compounds from fermentation broths and plant extracts, hoping to identify molecules that shaped flavor and aroma. Over decades, microbiologists followed its trail across various lactic acid bacteria species, finding it wherever lactic fermentation hummed along. Food technologists recognized its antimicrobial bite, particularly when dairy spoilage threatened profits. Scientific papers in the late 20th century linked the molecule to both natural processes in honey and cheese, and to modern interest in anti-infectives. Respect for the acid grew slowly, grounded mostly in hands-on lab work and food preservation trials, not hype.
3-Phenyllactic acid stands as a white crystalline solid, sometimes appearing slightly off-white if handled roughly. Chemical suppliers ship it under the names 2-Hydroxy-3-phenylpropanoic acid or PLA, tucked among stock for research chemicals, food preservatives, and rare bioactive agents. The molecule owes much of its attention to a warming, slightly floral aroma and a knack for stopping the growth of unwanted microbes. Humans taste it in honey and some sourdoughs. Most companies selling 3-phenyllactic acid offer it to scientists, fermentation technologists, and specialty food manufacturers looking to tap into its preservative edge. Not every chemical finds a place in daily production plants, but PLA’s natural occurrence gives it an edge over preservatives that nobody can pronounce.
PLA melts in the range of 117-119°C. Its solubility in water runs modestly low but jumps in common organic solvents like ethanol and acetone, making lab work straightforward. The structure features an aromatic ring linked to a lactic acid-like backbone, bridging flavor chemistry and organic synthesis. Acid dissociation constant (pKa) hovers around 3.88, giving PLA a bite not unlike vinegar but with a distinctly floral, honeyed aftertaste. Spectral fingerprints come in handy during quality control: strong signals in NMR, IR, and mass spectrometry make mislabeling difficult. For anyone handling jars in a lab or factory, PLA’s stability under cold, dry conditions keeps losses minimal, though moisture creeps in if seals slip.
Chemical manufacturers keep paperwork tight, labeling PLA with CAS number 828-01-3 and clearly marking containers with purity, water content, and production batch number. Certificates of analysis typically report a minimum purity of 98%, trace metals below 5 ppm, and moisture content below 0.2%. In research circles, suppliers flag optical rotation data, since chirality can twist biological performance. For those working under regulatory frameworks, safe handling symbols and storage instructions fill out each drum. Food-focused producers attach documentation on non-GMO status and identity-preserved supply chains, aware that food quality departments expect more than a sticker on a bag. Global trade names overlap, but most labs recognize “PLA” or “3-Phenyllactic Acid” on a bottle, and regulatory registration in the US and EU follows strict guidelines for safety, disposal, and accidental spill responses.
Industrial production of PLA heads down two main roads: biosynthesis and chemical synthesis. Traditional labs coax lactic acid bacteria like Lactobacillus plantarum or Streptococcus thermophilus to churn it out in rich culture broths, then recover product through acidification, filtration, and solvent extraction. Companies with the bandwidth to run fermentation often use food byproducts like whey or molasses, tuning parameters to favor phenyllactic acid over bland lactic acid. Chemists in organic synthesis prefer a more direct route, building PLA from phenylacetaldehyde using cyanide chemistry or hydride reduction, then running mild hydrolysis to liberate the final acid. Each approach has quirks: fermentation brings high purity with fewer synthetic byproducts, but lower yields and longer timelines; chemical synthesis works faster but introduces handling risks and extra purification steps. Any route still demands careful neutralization, crystallization, and drying — shortcuts lead to off-odors or chemical instability.
PLA’s backbone welcomes common organic tweaks. Chemists see the alpha-hydroxy acid as a handle for esterification, turning it into esters or amides that unlock new flavors, solubilities, or biological behaviors. One path adds an acyl group to the hydroxy, generating esters with higher volatility for fragrance or flavor work. Another tacks on hydrophilic groups for pharmaceutical delivery options. Oxidizers, under careful control, can cleave the aromatics for niche specialty acids used in deeper synthetic projects. Researchers see promise in coupling PLA to other bioactive structures, hoping for new antimicrobials or tissue-penetrating agents. Each step relies on classic organic reactions: acid catalysis, dicyclohexylcarbodiimide (DCC) for amide formation, or oxidative cleavage for ring modifications.
Nobody unfamiliar with lab nomenclature finds “2-hydroxy-3-phenylpropanoic acid” catchy, so markets usually stick with “3-Phenyllactic Acid” or simply “PLA”. Some older textbooks report “benzeneacetic acid, alpha-hydroxy-,” but this name caused confusion before more systematic rules came along. In food chemistry circles, “PLA” can refer to both D- and L- isomers, each with slightly different properties. Flavor industry documents occasionally list “phenyllactic acid” as an aromatic binder. Co-opting everyday language rarely lands for chemical names, so people in the know usually stick to PLA or 3-Phenyllactic Acid across scientific papers, patent filings, and supplier catalogs.
Handling PLA means sticking to well-known industrial hygiene standards: wear gloves, goggles, and lab coats. On contact, PLA can irritate skin or eyes. Dust from large volumes causes sneezing or mild throat irritation. Material safety data sheets (MSDS) flag its mild acidity and low volatility, so inhalation risks run low in properly ventilated spaces. Spilled crystalline PLA sweeps up with minimal fuss, but wet floors turn slick fast — plenty of safety managers learned that lesson the hard way. Environmental regulations push for collection and incineration over drain disposal. Regulatory authorities like OSHA and the European Chemicals Agency track workplace exposure limits and demand up-to-date training for plant staff. For shipments, transportation codes treat PLA as non-hazardous in moderate quantities, easing logistics but demanding accurate labeling and sealed containers to deter curious hands or accidental food contamination.
Food preservation leads the charge for PLA. Cheesemakers and yogurt factories find PLA naturally forming during standard lactic fermentation, slowing spoilage and fighting off invaders like Listeria and Bacillus. Artisanal bakers rely on lactic acid bacteria in sourdough cultures producing PLA to dampen mold growth and lend distinctive aroma. Outside food, researchers pursue PLA as a potential pharmaceutical agent, its knack for inhibiting harmful bacteria sparking trials in wound care and dental applications. Agricultural scientists explore its effect on post-harvest crops, where crop rot can wipe out months of work. Chemical manufacturers look at PLA’s role as a precursor for more advanced organic chemicals or as a specialty acid in complex syntheses. Startups tinker with PLA-based disinfectants for sensitive medical surfaces, where resistance to conventional chemicals mounts. Commercial growth still pivots on overcoming scale-up challenges — both affordability and regulatory approval remain hurdles in global markets.
Research teams worldwide dig into the biosynthetic pathways that let lactic acid bacteria make PLA. Genome editing tools, like CRISPR, enhance PLA yields and push new species toward optimized production. Analytical scientists fine-tune detection by mass spectrometry and high-field NMR, necessary for both food traceability and synthetic quality control. Interdisciplinary projects bring together fermentation experts, microbiologists, and bioengineers to open new uses—think active coatings for fresh fruit or probiotic strains for antibiotic-free livestock feed. Pharmaceutical scientists develop PLA derivatives, exploring how tweaks to the backbone might unlock new anti-inflammatory or anti-cancer properties. Chemistry departments model reaction pathways, searching for greener, cheaper synthetic routes that cut reliance on petroleum-based precursors. For every commercial batch, a pipeline of research fuels improvements in purity, functionality, and application.
Teams studying PLA’s safety profile go beyond food-grade testing, examining cellular, animal, and clinical outcomes. Oral toxicity studies in rodents report low acute toxicity, with minimal metabolic disruption even at high doses, though repetition and broader species coverage strengthen confidence. Food scientists look for allergenicity or off-flavors introduced by PLA at preservative concentrations, aided by taste panels and metabolic studies. Dermal and eye exposure studies rarely report significant irritation at practical exposure levels. Chronic exposure data lag behind, pushing for longer-term rodent studies and in vitro metabolic screening. Regulatory bodies require detailed breakdown of metabolites, since breakdown products can matter more than parent acid in sensitive populations. Ongoing surveillance, especially in countries permitting direct application in foods or supplements, keeps potential risks in check while flagging rare side effects for future review.
PLA’s trajectory depends on whether researchers and entrepreneurs bridge lab success with marketplace realities. Microbial fermentation, supercharged by gene editing and process engineering, promises sustainable production with lower carbon footprints compared to petrochemical routes. If everyday food manufacturers buy in, natural labeling gives them a competitive pitch as customers demand fewer synthetic additives. Advances in encapsulation and slow-release coating may push PLA into higher-value roles as an active agent in smart packaging or medical materials. Pharmaceutical pipelines could benefit as PLA derivatives show promise as preservatives in new drug formulations or as bioactive boosters when conventional antibiotics lose ground. Regulatory clarity, backed by strong toxicity and metabolic data, will steer adoption, especially in global supply chains hungry for reliable, safe antimicrobial agents. For innovators, PLA looks less like a single-molecule commodity and more like a versatile building block for next-generation products in medicine, food, and advanced materials.
Walk down the aisle in any supermarket, and the shelves burst with products claiming freshness. Keeping food clean and safe takes effort behind the scenes. One of the unsung players in the battle against spoilage is 3-phenyllactic acid. This compound doesn’t get flashy headlines, but its impact can be felt across several industries, from food production to medicine.
Wherever fermentation happens, the need for keeping out the wrong type of bug never goes away. Lactic acid bacteria produce 3-phenyllactic acid as a natural way to outcompete other microbes. This acid keeps spoilage molds and bacteria in check. For example, in cheese-making, the compound helps extend the product’s shelf life. The use of natural antimicrobials matters because it prevents the over-reliance on synthetic preservatives, which have raised health and environmental concerns over the years.
Research published in journals like Food Control and Journal of Dairy Science confirms that 3-phenyllactic acid can slow down or stop Listeria, Salmonella, and some strains of yeast and mold. Cheese factories and producers of fermented vegetables are using this to help meet consumer demand for foods without chemical preservatives. Seeing “No added preservatives” on a label has real value for many shoppers, and using 3-phenyllactic acid gives producers a way to provide that.
Interest in 3-phenyllactic acid goes beyond the kitchen. Researchers continue to explore its effect on antibiotic-resistant bacteria. Hospitals struggle with so many infections that don’t respond to traditional drugs anymore. In lab settings, 3-phenyllactic acid shows an ability to break down biofilms, those stubborn colonies that protect infections. The science isn’t yet ready for the doctor’s office, but every advance here helps keep standard antibiotics useful just a little longer.
Clinical scientists keep a close watch on compounds like this because antibiotic resistance won’t solve itself. The World Health Organization recognizes resistance as one of this generation’s main health threats. Every option, no matter how small it seems, adds to the arsenal.
Food waste drains money and resources, both at home and across the supply chain. Using 3-phenyllactic acid in packaging and storage could extend the life of bread, yogurt, and ready-made meals. Keeping food edible for longer means fewer truckloads get tossed, holiday pantries stay stocked, and the planet gets a break from excess production and landfill pressure. Agriculture and food industries both gain, especially as regulations push for safer, cleaner preservatives. For businesses, switching to safer methods can also mean better export prospects, as overseas markets become stricter about food additives.
The full potential of 3-phenyllactic acid isn’t tapped yet. Cost, production techniques, and scaling up from laboratory trials take investment. Small-scale producers struggle with access, and not every product reacts the same way to the compound. The challenge lies in tailoring its use for each product without messing up flavor or texture.
There’s no magic bullet for safer food or healthcare, but a naturally occurring acid like this brings practical benefits. Over time, with clear regulation, industry feedback, and continued research, it can help both food producers and medical teams deliver safer products and cut down on waste.
3-Phenyllactic acid crops up in conversations about food preservation and health supplements. Made by lactic acid bacteria in fermented foods, this organic acid sometimes appears in honey and even in kimchi. Its popularity comes from its ability to slow down the growth of some microbes, offering a natural option to keep foods fresh a bit longer. Lately, manufacturers have thought of adding it to foods as a preservative or using it in probiotic blends. Before tossing it into the shopping cart, it makes sense to ask if it really measures up on the safety front.
Plenty of current research on 3-phenyllactic acid sits at the intersection of microbiology and nutrition. Most of what I’ve read comes from university studies and technical journals. In the lab, this acid often passes toxicity checks. For instance, a 2020 study published in "Frontiers in Microbiology" showed that the compound didn’t harm cell cultures, even at concentrations higher than those found in fermented foods.
Beyond the petri dish, researchers have given small doses of 3-phenyllactic acid to rats and observed no liver damage, unusual behavior, or other warning signs. The doses in these experiments often far exceeded what people eat through naturally fermented products. So far, no strong evidence links this compound to allergies, cancer, or chronic medical problems.
The European Food Safety Authority (EFSA) and United States Food and Drug Administration don’t list 3-phenyllactic acid among their restricted additives. These agencies tend to set high bars, so their silence usually means a compound hasn’t raised alarms at current exposure levels. I haven’t seen it show up on recall notices or warning bulletins, either.
It’s important to point out that food science still works with a limited pool of large, long-term human studies. Researchers know quite a bit about lactic acid and its salts, but the body of work on this particular derivative isn’t nearly as deep. Most studies focus on bacteria or mice, and the handful that test on people look at foods like yogurt or kefir—so the intake is always part of a blend, not isolated.
One thing that sticks with me: some additives seem safe until they turn up everywhere, and then rare side effects come out. Take aspartame or artificial coloring—nobody flagged trouble at first, but looking back, many wish they’d dug a little deeper before sending them to market. It’s easy for a harmless compound to pick up a different story if it ends up in dozens of products at high doses.
If manufacturers want to scale up the use of 3-phenyllactic acid, more targeted research needs to happen. Testing should look at a range of age groups and health backgrounds, and studies ought to use pure forms, not just fermented foods. Independent trials, not just industry-sponsored ones, usually dig up more honest answers. Regulatory agencies should keep tabs on any changes in how it's used, watching for new evidence and erring on the side of caution when gaps in the data turn up.
As a shopper, the best move is to treat it the same way you’d treat any new food ingredient. If you're allergic to fermented foods, ask a doctor before trying supplements that list it. If you care about food purity, check for updates from food safety boards and nutrition journals. Stay curious and ask questions. That’s how real food safety gets stronger.
Food safety has always been a big deal, both for producers and for everyone who eats. Bacteria spoilage in bread, cheese, yogurt, and meat racks up huge losses and sometimes even lands on the evening news. 3-Phenyllactic acid, a natural compound found in honey and fermented foods, stands out as a real antibiotic workhorse. Scientists have tested it head-to-head against spoilage microbes such as Escherichia coli, Listeria monocytogenes, and Aspergillus molds. It wrecks their plans to grow, either by stalling their enzymes or poking holes in their membranes. Researchers in Japan and Europe saw milk and cheese last longer by days just by including it in the process. Fewer recalls, less food waste, and more peace of mind each time we bite into a sandwich.
Fermented foods often mean strong, healthy flavors and a gut-friendly boost. In most kitchens, folks rely on lactic acid bacteria to keep fermentation ticking. 3-Phenyllactic acid turns up as both an indicator and a guardian. Many strains of Lactobacillus plantarum and Lactococcus lactis churn it out naturally. In sourdough, kimchi, and other probiotic-rich snacks, it helps friendly flora fend off invaders. Honest, fresh sourdough feels different, inside and out, than bread lost to mold. When food scientists at Danish universities added 3-Phenyllactic acid to sour milk or cucumber brine, the numbers of “bad actors” plummeted while probiotics flourished. Living without belly aches or digestive complaints really adds up over time, especially for families watching their health.
Livestock, just like humans, don’t thrive on contaminated feed. Fungi growing in stored grains can kill profit and bring farms to their knees. Tests show that 3-Phenyllactic acid stops common feed mold including Fusarium and Penicillium before they pump out toxins. This means healthier animals, less medication, and food from animals raised without a pharmacy menu of antibiotics. Agricultural teams in China and Brazil reported firmer silage for cattle and stable grain for poultry when using this acid as a feed additive. That reduces dependency on chemical preservatives and helps address rising resistance to antibiotics—a problem global health bodies track closely.
With a steady push for food labels without tongue-twisters, 3-Phenyllactic acid lines up well. It forms in familiar foods—cheeses, yogurts, sourdoughs—that people already enjoy. Regulatory reviews in the EU and the US place it among the safer preservatives, especially compared to some synthetic options. This checks off the “clean label” box for food companies chasing both safety and customer trust. I’ve seen busy parents reach for products they know will be gentle on sensitive stomachs, and food producers lean into ingredients that don’t sound like a chemistry exam.
Many researchers believe 3-Phenyllactic acid can play a bigger role in both big food businesses and small-batch producers. Studies so far point to new uses in protecting shelf-stable goods, preserving freshness in prepared meals, and creating feed blends for organic livestock. Problems like mold growth and spoilage drag down profits, health, and food shelf stability. Investing in better fermentation techniques or working with natural food-additive suppliers helps address food safety and public health. As more companies and farmers turn to solutions that mesh with both science and local food traditions, benefits like those from 3-Phenyllactic acid stand to grow even further.
Anyone with a bit of lab or chemical storage experience understands the headache of chemicals degrading simply because someone cut corners on storage. Take 3-Phenyllactic Acid as a good example. This compound shows up often in industries ranging from food preservation to pharmaceuticals. What seems like a simple white powder at first glance turns into a real headache if stored in the wrong environment, especially once you deal with contamination, clumping, or even changes in chemical behavior. It isn’t just about following protocols for the sake of documentation; it’s about protecting both the product and the people working with it.
Sticking 3-Phenyllactic Acid on a random shelf in the supply closet isn’t the best idea. This acid stays reliable around 2-8°C—a common fridge temperature. Speaking from a stint managing university lab stocks, fluctuations near room temperature meant opening a bottle to find caking or crystals sticking together. Those small changes make dosing in precise experiments tougher and can even disrupt measured results. Keeping a dedicated fridge just for sensitive reagents like this one saves money and time in the long run.
In plenty of working labs, someone always grabs a jar “just for a second” and then puts it back with the lid loose. Moisture gets in, and suddenly, there’s a sticky mess or—worse—a sample now changed at the molecular level. 3-Phenyllactic Acid draws water from the air, which means a tight lid is a must. It goes beyond mold prevention; any trace of water shortens shelf life and can ruin batches. Always make sure bottles and vials snap or screw shut with a good seal. Desiccators, those boxes filled with drying agents, also come in handy, especially in shared storage situations where fridge doors swing open fifty times a day.
Repeated exposure to light, especially sunlight or harsh lab lights, does no favors for 3-Phenyllactic Acid. UV kicks off breakdown reactions that you can’t always see with your eyes, but definitely notice the next day when results are off. Amber glass vials or structured containers do a far better job than cheap plastic tubes. Avoid windows, windowsills, or clear bags. Even ambient light inside a fridge can matter over several months.
Faded ink, missing expiration dates, mystery containers… they’re disasters waiting to happen. Every package containing 3-Phenyllactic Acid should list the date received, lot number, and shelf expiration date. More than once, proper labeling saved my team from using an expired batch, risking both research and personal safety. Labs tracking inventories with spreadsheets or simple barcode systems cut down drastically on misplaced or mishandled samples.
Minor investments—tinted bottles, reliable labeling pens, steady refrigeration, and those silica gel packs tossed inside—pay off more than fancy storage cabinets or outsourcing chemical management. And if the chemical ever gets repacked or aliquoted, each smaller bottle deserves the same care as the original container. Ignoring storage advice turns one mistake into a chain reaction, reaching from supply rooms to production lines or clinical tests.
Peer-reviewed sources, like the Sigma-Aldrich chemical catalog or the Material Safety Data Sheets (MSDS) published by global suppliers, echo every step listed above. They all agree on the importance of cold, dry, and dark storage, and flag the risks of careless handling for both safety and effectiveness. The scientific literature also highlights cases where improper storage led to misleading research or contamination scares.
Bad storage isn’t just inconvenient; it can turn a helpful tool into a hazard. People underestimate the rippling costs of wasted chemicals, compromised tests, and even recalls. Teach best practices to everyone in a workspace, invest in a few basic supplies, and pay close attention to how often fridges and cabinets get checked and cleaned. Each step supports both science and safety.
3-Phenyllactic acid doesn't usually steal headlines, but you’ll spot it on ingredient lists for a reason. It shows up mostly in fermented foods, dietary supplements, and cosmetic formulas. This molecule, produced naturally by some lactic acid bacteria, brings both preservation and potential health benefits.
Fermented dairy like yogurt and cheese, sourdough bread, and even kimchi tend to have this acid at low concentrations. It plays a key role in keeping spoilage microbes at bay. Beyond preservation, there’s ongoing research into its function as an antimicrobial that might support digestive health—although grand claims often outpace solid evidence.
In supplements or cosmetics, you'll find it added more for its targeted antimicrobial effects. Formulators lean on it to curb unwanted bacterial growth, especially in products with water or delicate organic content.
Naturally, the amount varies. Cheeses and yogurts often contain 3-phenyllactic acid from 5 up to 50 mg per kilogram due to milk fermentation. Sourdough bread can register even lower; measurements often land under 10 mg/kg. These concentrations result from traditional processes not specifically tailored for boosting this compound, but rather as a byproduct of bacterial action.
Cosmetic producers take a different tack. Here, you'll see concentrations between 0.05% and 0.2%, depending on the formula and product goals. These levels reflect calculations based on both safety and functional need—nobody wants skin irritation, so companies play it safe with the lowest effective strength.
Dietary supplements sometimes skirt higher, guided by science but more so by regulatory thresholds and a bit of commercial optimism. Most capsules or powders targeting gut health don’t top 100 mg per serving. The European Food Safety Authority has kept an eye on intake through normal food use and hasn’t flagged health risks, as actual exposure looks pretty modest next to common food acids. Still, reliable long-term studies for regular supplemental intake remain scarce.
Looking around the industry, you see wide variation. Product intent matters. Food safety relies more on lactic acid itself, with 3-phenyllactic acid playing a backup role. People making “clean label” products sometimes experiment with more, but keeping food taste and regulatory standards in check puts a ceiling on how much they'll add.
Cosmetic chemists and supplement formulators run tests to find their sweet spot—enough to stop bacterial growth, below the line where irritation or side effects show up. Consumers, especially those sensitive to phenylalanine or with metabolic conditions, get left out if labeling isn’t clear or intake climbs higher. Better transparency would definitely help, and regular third-party testing adds an extra guardrail.
Tracking more real-world product data would give everyone more confidence—consumers and manufacturers included. It makes sense to keep pushing for clear dose guidelines in all uses, and for more human studies tracking cumulative daily intake. A future where shoppers know exactly how much 3-phenyllactic acid they get from cheese, supplements, or body lotion isn’t out of reach, and clearer policies could make it reality.
| Names | |
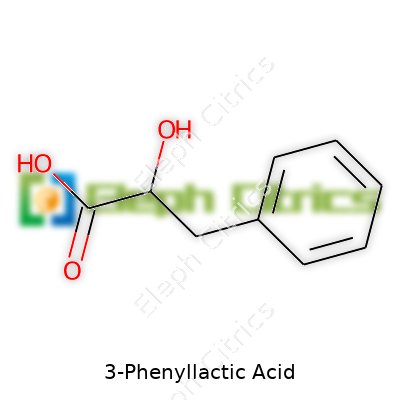
| Preferred IUPAC name | 2-hydroxy-3-phenylpropanoic acid |
| Other names |
Phenyllactic acid PLA 2-Hydroxy-3-phenylpropanoic acid 3-Phenyl-lactic acid α-Hydroxy-β-phenylpropionic acid |
| Pronunciation | /ˌθriːˌfɛnɪlˈlæktɪk ˈæsɪd/ |
| Identifiers | |
| CAS Number | 828-01-3 |
| Beilstein Reference | 1206062 |
| ChEBI | CHEBI:16961 |
| ChEMBL | CHEMBL59593 |
| ChemSpider | 12590 |
| DrugBank | DB04160 |
| ECHA InfoCard | ECHA InfoCard: 100.011.790 |
| EC Number | 3.1.1.88 |
| Gmelin Reference | 89801 |
| KEGG | C00855 |
| MeSH | D017783 |
| PubChem CID | 98158 |
| RTECS number | SL8410000 |
| UNII | 7JM13PIN1A |
| UN number | UN2811 |
| Properties | |
| Chemical formula | C9H10O3 |
| Molar mass | 166.18 g/mol |
| Appearance | White to off-white crystalline powder |
| Odor | odorless |
| Density | 1.24 g/cm3 |
| Solubility in water | Slightly soluble in water |
| log P | 1.18 |
| Vapor pressure | 0.0000133 mmHg at 25°C |
| Acidity (pKa) | 3.62 |
| Basicity (pKb) | 2.97 |
| Magnetic susceptibility (χ) | -7.59×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.553 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.97 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 157.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -537.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1749.1 kJ/mol |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H317: May cause an allergic skin reaction. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| Flash point | > 164.6 °C |
| Lethal dose or concentration | Lethal dose or concentration (LD50, Oral, Rat): 4640 mg/kg |
| LD50 (median dose) | LD50 (median dose): **810 mg/kg (rat, oral)** |
| NIOSH | DFN8R69L9T |
| PEL (Permissible) | Not established |
| REL (Recommended) | 10 μg/kg |
| Related compounds | |
| Related compounds |
Lactic acid Mandelic acid 3-Phenylpropionic acid 4-Phenyllactic acid 3-Hydroxy-3-phenylpropionic acid |