Long before the era of high-speed discovery, 8-hydroxyquinoline caught the attention of chemists due to its intriguing structure and strong chelating ability. Betttering its parent compound with the addition of citrate, researchers shaped a legacy that linked organic chemistry to practical needs in medicine and industry. The use of 8-hydroxyquinoline citrate in various forms started to pick up in the early to mid-20th century, driven largely by the pharmaceutical sector and later by fields like agriculture and analytical chemistry. During these decades, scientific literature started to recognize this compound as a reliable and versatile player, with early patents and journals describing its preparation and uses, far before regulatory frameworks standardized chemical quality or labeling.
8-Hydroxyquinoline citrate stands as a salt combining 8-hydroxyquinoline’s chelating powers with citric acid’s solubility benefits. This pairing allows for easier handling and improved bioavailability compared to the parent molecule. Solid at room temperature, usually pale yellow to off-white, this product dissolves more readily in water than its free base, an important advantage in some medical and industrial applications. Its broad functional profile reaches from use as a preservative in cosmetics to metal ion control in laboratory assays, and the reliability of the product often stems from sourcing with consistent assay, water content, and purity, factors that matter both for bench chemists and end users.
In daily lab work, the physical constants make a real mark: 8-hydroxyquinoline citrate typically comes as a crystalline or powdery substance, sometimes slightly hygroscopic, which matters a lot for storage and weighing accuracy. Its melting point ranges depending on purity, and the compound holds up fairly well under normal storage conditions away from strong acids and bases. The molecule’s ability to act as a ligand, forming complexes with a broad spectrum of metal ions, drives both its value and its chemical reactivity. This trait ties back to the presence of the hydroxyl group at the 8-position and the nitrogen atom in the quinoline ring, both crucial for interactions with metals. Under routine work, chemists notice its faint, sometimes slightly bitter odor; those who have handled various quinoline compounds will remember this characteristic.
Quality control professionals check important characteristics including assay percentage, pH of a standard solution, content of impurities (like sulfate or heavy metals), moisture, and sometimes UV-visible absorption values. Labeling demands care; regulatory agencies expect hazard communication, correct identification (using CAS number and recognized synonyms), and manufacturer details. Labels commonly list both synthesis and expiration dates, storage precautions, and appropriate hazard codes. All of this supports both traceability and safe use throughout the chemical’s life cycle.
Manufacturers produce 8-hydroxyquinoline citrate by direct neutralization of 8-hydroxyquinoline with citric acid, often in aqueous or ethanol-water solutions. The method involves carefully controlling pH and temperature, ensuring full dissolution, then precise crystallization to yield a consistent product. Filtration, washing, and drying steps all contribute to high-purity material. Operators often rely on simple, robust routes rather than multi-step synthesis, which keeps costs in check and yields predictable batches. In research settings, smaller quantities can be prepared on the bench with standard glassware, given proper ventilation and personal protective equipment.
The basic structure offers multiple handles for further reactions. Nitration, sulfonation, and alkylation can modify the base quinoline ring, while the citrate component can sometimes serve as a leaving group in certain transformations. Its chelating power lets it extract and immobilize metal ions, making it useful in analytical labs for metal ion detection or separation. Researchers often use it as a starting point for synthesizing more complex pharmaceuticals, pesticides, or dyes, attending closely to its reactivity profile. Routine use in complexometric titration reveals its strong binding with metals, producing colorimetric responses visible even to the naked eye.
In catalogs and research papers, 8-hydroxyquinoline citrate often appears as oxyquinoline citrate, oxine citrate, or 8-quinolinol citrate. Other trade names reflect the manufacturer or targeted application. Consistency across naming conventions matters for inventory tracking and scientific communication, especially in global supply chains where mislabeling or confusion can lead to costly mix-ups.
Safety standards for 8-hydroxyquinoline citrate require gloves, goggles, and standard ventilation during handling, especially at scale. Its low to moderate toxicity profile translates to the need for good laboratory hygiene and secure storage away from food, feeds, and incompatible materials. Organizations such as OSHA and local equivalents prescribe permissible exposure limits, although these rarely come into play given the quantities in most non-industrial settings. Waste disposal must comply with local regulations, and spill response plans keep accidental releases in check. Training and up-to-date safety data sheets keep both new and experienced users informed.
Hospitals, clinics, and diagnostic labs have found a steady place for this compound in antibacterial preparations and wound dressings, owing to both its chelating action and mild antiseptic properties. In agriculture, 8-hydroxyquinoline citrate curbs fungal growth on plants and cuttings. Water testing labs utilize its ability to bind trace metals. Industrial players utilize it for preserving cutting oils, inks, and even some types of photographic chemicals. Always, the value stems from reliability and predictable performance, giving assurance to users from field workers to analytical chemists.
Ongoing research targets improvements in formulation, delivery, and environmental compatibility. Novel hybrid compounds and slow-release forms are being explored for both medical and agricultural applications. Analytical chemists experiment with derivatives in search of increased selectivity or sensitivity for specific metal ions. R&D teams look to reduce byproduct generation and improve synthesis routes to decrease costs and lower environmental impact, echoing the broader movement toward green chemistry.
Toxicity studies on 8-hydroxyquinoline citrate focus on both acute and chronic exposure. Oral and dermal toxicity sits in the low to moderate range, but workers who repeatedly handle it without proper precautions face risk of skin and respiratory irritation. Studies on aquatic toxicity suggest moderate persistence depending on local water conditions. Regulatory agencies build safe use guidelines from both independent and sponsored studies; information from these sits at the core of modern handling standards. Chemists and product users alike pay careful attention to data on bioaccumulation and metabolic breakdown, a practice driven both by regulation and a strong sense of responsibility in the scientific community.
The outlook for 8-hydroxyquinoline citrate stands linked closely to advances in detection, prevention, and sustainable manufacturing. Refined synthesis minimizes waste and energy use, while deeper understanding of its environmental impact drives push towards biodegradable analogs or improved recyclable processing. In medical settings, work continues on better methods of delivery to infected or inflamed tissues, with combination therapies drawing interest. As markets expand in Asia and Africa, demand grows for affordable, effective, and shelf-stable antimicrobial products, keeping this compound in the sights of both researchers and producers. More open access to research will accelerate innovation, ensuring broader and safer uses across many fields.
8-Hydroxyquinoline Citrate shows up in a lot more places than most folks realize. It’s a derivative of 8-hydroxyquinoline, which chemists identified back in the early 1900s for its metal-binding abilities. Today, it takes on roles that touch everything from agriculture to medicine.
One place you’ll find this compound hard at work is agriculture. Farmers lean on it to fight fungi and bacteria that can devastate crops. I’ve seen local citrus growers use it to keep fruit molds under control after harvest. Post-harvest rot chews through profits, and growers get desperate for safe options. This is where 8-Hydroxyquinoline Citrate steps in: it stops fungal growth, protects supply, and keeps products safe for shoppers. Some flower growers even rely on it to extend vase life by controlling the bacteria and algae that mess up clean stems.
Labs treat this compound as a basic tool for separating and identifying metals. Chemists call it a “chelating agent,” which means it grabs onto metal ions, forms stable complexes, and helps pull out trace metals from solutions. Water treatment plants use variations of 8-hydroxyquinoline to test for copper and iron contamination, levels that matter for both machines and people. The compound’s effectiveness comes from its ability to grab heavy metals that slip past weaker treatments—something underscored by the high standards in environmental labs.
Some researchers look at 8-Hydroxyquinoline Citrate as a possible tool against skin infections and bacterial growth. In my experience, the chemical has long attracted attention for antiseptic potential. Early studies, especially from the 1970s and 80s, explored it as a topical agent for wounds and ulcers. Success comes down to its ability to block enzyme activities in harmful microbes. Hospitals and clinics don’t use it as widely now as they did fifty years ago, since stronger and safer antibiotics have appeared, but some ointments and disinfectants still owe their benefits to molecules in the hydroxyquinoline family.
No conversation about a compound like this ignores safety. Even though 8-Hydroxyquinoline Citrate has uses, folks working with it face risks from both skin exposure and inhalation. The World Health Organization pays close attention to substances in this chemical family, especially when people could add them to food or water accidentally. The European Union regulates its use in agriculture via strict limits, keeping residues well below what’s considered harmful. Scientists continue to study possible links to environmental impact—it sticks around in soil and water longer than many natural compounds, and there’s concern about how it affects small water bugs and soil life. Checking labels and following rules goes a long way in keeping people and ecosystems safe.
If society wants to keep getting value from 8-Hydroxyquinoline Citrate, a few things help. Teachers and scientists should spread awareness among farmers, florists, and lab workers about the tiny doses that matter and the importance of proper handling. Strong packaging, locked chemical cabinets, and regular audit trails all make a meaningful difference. Medical researchers now seek gentler substitutes for tough old antiseptics like this one. As regulations change and more information emerges, the best approach keeps combining curiosity, caution, and respect for what these complex chemicals can do.
People often ask about the safety of different chemicals used in health products, industrial processes, or research settings. 8-Hydroxyquinoline Citrate pops up in these conversations, known for its role as an antiseptic and preservative. Some doctors prescribe it for gut infections. While it can be effective, there’s a fair amount to consider about the risks tied to its use.
Most people tolerate 8-Hydroxyquinoline Citrate without major trouble if they stick to recommended doses. The most common complaints involve the gut. Nausea, stomach cramps, and diarrhea have been reported. Having worked in a pharmacy for several years, I’ve seen folks come in with milder stomach issues after a run of antibiotics or similar substances. Similar patterns happen here, probably because this chemical messes with the balance of bacteria in the intestines.
The next thing that pops up is headaches. This side effect isn’t unique to this substance, but people using products with 8-Hydroxyquinoline Citrate sometimes mention it. A few also talk about dizziness or a general feeling of being off-balance.
Rare doesn’t mean never. Sometimes a person reacts more strongly. Allergic reactions can happen, leading to skin rashes, itching, or swelling of the face or mouth. If breathing becomes difficult, it’s a medical emergency and people need to get help right away. From what I’ve seen, allergies to this compound are less common than with penicillin or sulfa drugs, but they are not unheard of.
Another concern: 8-Hydroxyquinoline Citrate can sometimes affect the nervous system. There have been documented cases—mainly when people used it for a long time—of tremors, muscle weakness, or numbness in the hands and feet. Some sources, including the World Health Organization, note the risk of something called subacute myelo-optico-neuropathy (SMON), a serious condition that hit some countries hard in the mid-1900s after this chemical saw wide use. Symptoms include pain, tingling, movement issues, and sometimes vision problems.
Kids, folks with kidney or liver problems, and older adults need to be especially cautious. Their bodies process drugs and chemicals differently, and this can bring on side effects more easily or make them worse. Anyone with a history of nervous system problems should also talk to a healthcare provider before taking anything containing this chemical.
Most risks can be managed by using the product as directed. Reading the label, sticking with proper dosing, and letting a pharmacist or doctor know about other medicines or allergies can close many gaps in safety. Products bought outside regulated pharmacies might carry a bigger risk since it’s harder to know what’s really inside.
Over the counter, this chemical shows up in a handful of topical and oral products. If somebody develops gut issues, headaches, allergic signs, or any unusual nervous symptoms, it’s smart to get it checked out. Most of the trouble can be avoided with careful attention and reporting side effects early to a healthcare professional.
For anyone worried, there are often other options with fewer risks. Healthcare providers can offer guidance based on a person’s health history and current medications. Staying informed, watching for early warning signs, and trusting gut instincts go a long way toward staying safe.
8-Hydroxyquinoline Citrate comes up in medical and veterinary circles mainly as an antimicrobial. It enters real conversations when someone faces bacterial or fungal infections that don’t bow down to typical treatments. Doctors know its value. It’s not a supplement or a casual fix-it remedy; this is a substance with a specific science supporting its role against certain microbes.
The biggest mistake people make with compounds like this comes from self-direction. Folks see positive headlines online or see it mentioned in forums—then go all-in, guessing at doses. Mistakes can follow. Real harm too. That’s where prescription comes in. A professional weighs several things before suggesting a dose, like body weight, the severity of infection, and other medicines in play.
Someone dealing with kidney or liver problems faces extra concerns. The body processes medications through these organs; so if something isn’t working as it should, guessing at a dose can be more dangerous than helpful. Every pharmacy note and pharmaceutical text flags these issues for a reason. Ignore them and pay the price with side effects or outright toxicity.
Swallowing a prescribed tablet with water, preferably after a simple meal, lines up with most guidance I’ve seen. This step helps limit stomach upset, which medicines in this family can provoke. Crushing tablets or mixing powders without direction hasn’t just led to missed results—sometimes to worse troubles when absorption shifts in unpredictable ways.
Drinking extra fluids makes sense too. Speak with any pharmacist long enough, and you’ll hear story after story about dehydration compounding medicine issues. Good hydration keeps your kidneys moving toxins out and limits risks from any build-up of unexpected byproducts.
People report nausea, headaches, and skin reactions from this medication. I remember a veterinary colleague mentioning that dogs treated for fungal skin problems sometimes scratched even more than before—later traced back to allergies or overdosing. These signals need immediate attention. Ignoring them wastes precious time and comfort.
Antibiotics and antifungals always invite a conversation about resistance and superinfections. Overusing drugs creates tougher bugs. That’s not internet fear-mongering—it’s played out in hospitals across the world. The lesson I take is not to demand antibacterial medicines for every cough or rash. Let the findings rule the decision, not panic or hope.
Self-treating with 8-Hydroxyquinoline Citrate invites trouble. A healthcare professional understands not only how much and how long but also the particular risks in your body and daily routine. This medicine sometimes interacts with other drugs—especially those affecting liver enzymes—potentially leading to weakened results or new side effects.
Even with older, well-established medications, science changes as new studies arrive. That’s why real doctors and pharmacists keep referring to contemporary clinical guidelines, even with drugs they've dispensed for years. Home diagnosis and unsupervised use only erase any safety net.
Stick to the prescribed plan. Avoid food and over-the-counter pills that could interfere, unless specifically cleared by your medical provider. Keep an eye out for unexpected symptoms and flag these sooner than later. Follow-up visits often catch early warning signals that an individual might miss at home.
With the right input from healthcare experts and consistent, practical habits, treatments like 8-Hydroxyquinoline Citrate can deliver reliable results without unnecessary risks. These steps don’t just reduce guesswork—they raise the odds of a full, unhindered recovery.
People have used 8-Hydroxyquinoline compounds for decades, mostly to kill germs or fungi, either in labs or as part of treatments. You spot it sometimes in topical creams and disinfectants, and even old textbook pages mention it as a preservative or sanitizer. The citrate form shows up because it dissolves better, so you get more out of a dose.
Safety always gets questioned once something has a medical or industrial purpose, especially if there's a chance people will apply it more than once or keep using it. Many antimicrobials lose their shine once someone studies them long-term. We saw this happen with other chemicals that seemed harmless at first, then turned up problems after enough time passed.
Lab studies show 8-Hydroxyquinoline compounds can be toxic to cells at higher concentrations. They disrupt cellular metal balance—a function that microorganisms rely on, but so do our own bodies. Copper and zinc, for example, are crucial for human brain and nerve health. Studies on rodents show that long-term dosing affects the liver and kidneys. Researchers worry about possible neurotoxic side effects, meaning harm to the brain or nerves if taken for too long or at high doses.
There’s not a clear pile of published studies tracking real-life people on long-term regimens of 8-Hydroxyquinoline citrate. Medications containing similar molecules, like clioquinol, caused outbreaks of SMON (Subacute Myelo-Optic Neuropathy) in Japan decades ago, which doctors later blamed on unexpected neurotoxicity, especially with chronic exposure. No one wants a repeat of that.
Short-term use in small, controlled amounts—think skin cream applied for a few days—shows fewer problems. If someone swallows it regularly, or applies it all over for weeks at a time, the story could change. Regulators in the US and Europe draw hard lines for acceptable daily intakes, and nobody in mainstream medicine recommends it routinely anymore.
The stuff kills germs, no question. Hospitals sometimes turn to 8-Hydroxyquinoline when battling stubborn fungi on surfaces. For wipes or hand rubs, most risk comes from people accidentally swallowing it or using it with damaged skin. If you're thinking about beauty products or long-term treatments, risks swing higher, especially if the product is left on the skin and not washed off.
Our bodies handle stress and toxins in small amounts, but have limits. Relying on an antimicrobial that disrupts cellular metals over months or years poses unpredictable risks. The scientific advice says err on the side of caution unless a qualified healthcare professional specifically prescribes or recommends something containing it for a short span.
People need answers based on solid evidence, so long-term, controlled human studies matter. Until those exist, anyone using this type of product long-term should talk to their doctor and be wary of internet promises or cosmetic trends. Checking ingredient lists, knowing what gets absorbed, and looking for alternative solutions with established safety records makes more sense than gambling with uncertain science.
Chemicals like 8-Hydroxyquinoline citrate serve a purpose, but overuse or unknown exposure turns safe into risky. Transparency and real-world studies can turn vague warnings into concrete advice, so demanding more public data helps protect everyone.
8-Hydroxyquinoline Citrate often turns up in conversations about antimicrobial and metal chelation treatments. In human health, folks may see it prescribed for infections or as part of treatments that manage certain heavy metal poisonings. The public doesn’t tend to hear about this name at their pharmacy counter much, but with the spread of alternative therapies and experiments in integrative medicine, it pops up. Knowing the way this compound acts in the body shapes how it interacts with other medications. As someone who’s spent years combing through patient histories and drug information, I know one thing for certain: it’s easy to underestimate the power drug interactions wield.
Many people juggle multiple prescriptions, not just over-the-counter pills but herbal supplements or vitamins as well. This juggling act can lead to trouble. Interactions may spark new side effects, lessen a medicine’s punch, or push levels up high enough to risk toxicity. Looking back at some tough cases in clinic, I’ve seen patients who didn’t realize two small pills could trigger a big medical storm. This isn’t theoretical — it’s real life, and missing the signs can land someone back in the ER.
This compound acts by binding to metals in the body, which underpins its main uses. For those on supplements containing iron, calcium, magnesium, or zinc, this means 8-Hydroxyquinoline Citrate could latch onto those minerals and keep their absorption low. A person working to fight off anemia or replenish electrolytes from another diagnosis might not get the full effect of their prescribed supplements. Even with a balanced diet, these interactions matter.
Antacids often contain these minerals, and mixing antacids with 8-Hydroxyquinoline Citrate reduces the impact of both. Anything that slows stomach emptying or alters acidity could cause this chelating agent to stay in the system longer, increasing risk of side effects like gut pain or nausea. From cases I’ve read, one missed detail here can mean unnecessary discomfort for the patient.
Some antibiotics, especially tetracyclines and fluoroquinolones, show weakened absorption if taken too close to any chelating agent. Someone treating a tough infection with one of these antibiotics might not see full results if 8-Hydroxyquinoline Citrate competes for attention in their stomach. As a result, doctors generally space these drugs apart by several hours.
Reports also suggest that 8-Hydroxyquinoline Citrate can interact with medications used in managing seizures, blood pressure, or even heart rhythm. Drug metabolism depends on liver enzymes, and chelating agents sometimes interfere with this pathway, nudging drug levels higher or lower than planned. From a pharmacist’s point of view, these changes mean a once-stable treatment could become unpredictable.
Doctors and pharmacists stress keeping a complete medication list. Being upfront about everything taken — prescription, over-the-counter, herbal or vitamins — sidesteps many surprises. After all, gaps in electronic health records often exist, and nobody wants an interaction that could’ve been caught. Good communication builds safer care.
Spacing out the timing of doses lowers risk. Taking supplements or certain antibiotics a couple hours apart from 8-Hydroxyquinoline Citrate makes sense. Regular lab checks for anyone at risk of low minerals or shaky drug levels can pick up problems early. Patients who bring in questions get more targeted advice than those who just roll with the default.
In my own experience, slowing down and checking new combinations is worth the extra few minutes. Patience and a full view of what someone puts into their body helps everyone get better results, rather than letting stubborn interactions win.
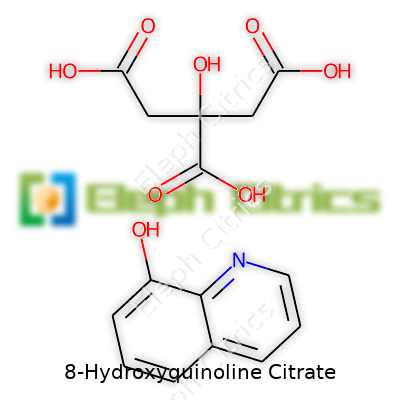
| Names | |
| Preferred IUPAC name | 2,3-Dihydroxypropane-1,2,3-tricarboxylic acid; 8-quinolinol |
| Other names |
Chinosol Oxyquinoline citrate 8-Quinolinol citrate |
| Pronunciation | /ˌeɪt haɪˌdrɒk.siˈkwɪn.əˌliːn ˈsɪt.reɪt/ |
| Identifiers | |
| CAS Number | 7048-44-6 |
| Beilstein Reference | 120873 |
| ChEBI | CHEBI:38720 |
| ChEMBL | CHEMBL1201127 |
| ChemSpider | 22060123 |
| DrugBank | DB05003 |
| ECHA InfoCard | 100.142.432 |
| EC Number | 242-285-4 |
| Gmelin Reference | 412176 |
| KEGG | C07442 |
| MeSH | D019226 |
| PubChem CID | 5460635 |
| RTECS number | VA0350000 |
| UNII | 122P0E6E3X |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C18H15N3O7 |
| Molar mass | C18H15N2O7 |
| Appearance | Pale yellow to yellow crystalline powder |
| Odor | Odorless |
| Density | 0.7 g/cm3 |
| Solubility in water | Soluble in water |
| log P | -1.1 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa 9.9 |
| Basicity (pKb) | pKb ≈ 6.1 |
| Magnetic susceptibility (χ) | -74.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.62 |
| Viscosity | Viscous liquid |
| Dipole moment | 8.53 D |
| Pharmacology | |
| ATC code | D08AX08 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS labelling of 8-Hydroxyquinoline Citrate: "GHS07, Warning, H302, H315, H319, P264, P270, P280, P301+P312, P305+P351+P338, P332+P313, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | NFPA 704: 2-1-1 |
| Flash point | > 195°C |
| Lethal dose or concentration | LD50 (rat, oral): 1200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1150 mg/kg |
| NIOSH | LM2975000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for 8-Hydroxyquinoline Citrate: Not established |
| REL (Recommended) | 150 mg |
| Related compounds | |
| Related compounds |
8-Hydroxyquinoline 8-Hydroxyquinoline sulfate 8-Hydroxyquinoline hydrochloride Quinoline Quinine Clioquinol |