Aluminum citrate didn’t just pop up out of nowhere. Scientists started looking into it seriously in the early 20th century, after figuring out the way that aluminum could bond with citric acid. This work started out in university labs, usually where researchers had both curiosity and enough grant money to go beyond baking-soda chemistry. Some of the early interest grew from studies on water treatment and medicine. Over time, demand expanded as folks realized this compound could show up in pharmaceuticals and industry. Universities wrote about aluminum citrate as folks started to see potential in its chelating skills and possible use in everyday products. By the 1980s, aluminum citrate no longer belonged just to researchers in white lab coats—it started entering water treatment protocols and dietary supplement discussions, even as the safety debates kept going.
You don't need to be a chemist to notice that aluminum citrate keeps popping up in niche industries. It’s a salt made by reacting aluminum with citric acid, and it’s labeled under several different names depending on who’s marketing it and how it’s intended to be used. Watch for it in laboratories, water purification setups, or in some health supplements, though strict regulation applies there. Pharmaceutical companies have tagged onto its potential for binding metals within the body, while water treatment operations lean on its chelating ability to grab onto impurities.
In its solid state, aluminum citrate usually appears as a white to off-white powder, sometimes with a faint granular texture. It’s pretty soluble in water, thanks to those multiple carboxylate groups on the citric acid part. Drop some in a beaker and it dissolves without a fuss, though it doesn’t do the same in ethanol. On the chemical front, it stands out for its ability to form stable, soluble complexes with various metal ions. The structure can get complicated, as each aluminum ion typically coordinates with more than one citrate molecule. Researchers have tracked this with X-ray crystallography and NMR, and they found a tangle of bonds that make it flexible for industrial settings.
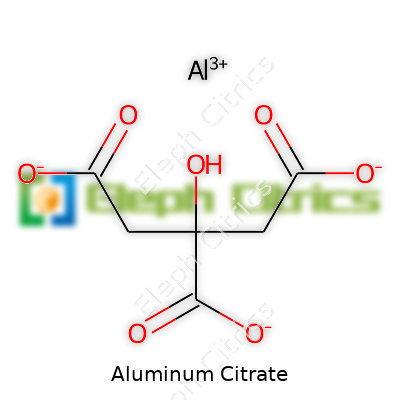
Specs matter, not just for the sake of paperwork but because impurities can gum up whole production lines or bring about unwanted reactions. Chemists running quality control tests check for things like heavy metal content, pH in solution, water insoluble matter, and particle size. Purity often stays above 98%. If you look at commercial labeling, you’ll spot fields that list molecular formula (usually Al3(C6H5O7)3), molecular weight, storage recommendations, and shelf life. Each batch might have its own lot number to keep things traceable, and regulatory paperwork demands details on handling precautions due to concerns about dust inhalation or contamination.
Making aluminum citrate usually starts with citric acid and aluminum source—frequently aluminum hydroxide or aluminum sulfate. The process calls for dissolving citric acid in deionized water, then slowly mixing in the aluminum compound under controlled pH. The resulting mixture is stirred vigorously until reaction finishes, then filtered to remove unreacted solids. Afterward, the solution gets concentrated and dried, often under reduced pressure to cut back on heat breakdown. Some manufacturers tweak conditions—temperature, pH, concentration—to control the final crystal structure, purity, and flow properties of the powder. On the safety side, working with fine powder means dealing with dust controls and proper ventilation.
One reason aluminum citrate attracts specialists is its knack for forming complexes. It grabs onto transition metal ions, which helps in both laboratory separations and certain detoxifying medical applications. In water treatment, aluminum citrate can bind heavy metals and allow for easier removal during filtration. Some teams experiment with modifying aluminum citrate by adding functional groups or exploring substitution reactions, aiming for compounds with tailored chelating profiles. Experiments with heating or varying pH can coax out different hydrate forms or polymorphs, which might slightly tweak solubility or reactivity, letting folks pick the blend best suited for their process.
Across suppliers and scientific literature, you’ll come across a handful of different names for aluminum citrate. Some call it “aluminum tricitratum”, “tricitrat Aluminium”, or simply “Aluminum(III) citrate”. Pharmaceutical and chemical supply companies might throw catalog numbers and product codes on the label, but the underlying compound stays the same. These synonyms mostly come from attempts to standardize names internationally, with organizations like IUPAC and CAS giving their own systemized touch.
Most people working hands-on with aluminum citrate know it’s generally not the most dangerous of chemicals, but safety isn’t negotiable. Breathing in the fine powder isn’t great for lungs, so proper masks are a must. Anyone handling it in bulk wears gloves and eye protection, and ventilation systems get checked on the regular. Regulatory agencies in Europe and North America classify it as a chemical for controlled industrial or research use, not something for casual handling or food-grade applications without rigorous testing. Waste disposal follows hazardous material protocols to prevent environmental contamination, and companies document any accidents instantly. Some guidelines highlight aluminum’s role in neurological disorders, which keeps the use of this compound tightly overseen where ingestion or bodily contact might occur.
Aluminum citrate finds homes in specialized corners of industry. In water purification, it acts as a chelating agent, snagging heavy metal ions so they can be filtered out of municipal and factory wastewater. Pharmaceutical formulations sometimes lean on its ability to bind metals in the gut or bloodstream, often in treatments for metal poisoning. Dentists and manufacturers of oral care products look at its astringent and binding properties—though there’s debate about long-term safety here. A few researchers tested it as a catalyst in organic synthesis, using its unique scaffold to speed up certain reactions. On rare occasions, it’s evaluated as a possible food additive, but most authorities only approve it under strict maximum limits and clear labeling.
R&D teams haven’t let go of aluminum citrate. Some focus on improving synthesis for better yields or less waste, chasing smarter process controls and greener chemistry. Others study its interactions with biological molecules, hoping to expand its use in medicine—from targeted drug delivery to new forms of chelation therapy. Many groups benchmark its chelating ability against other compounds to see if there are efficiencies to unlock in fields from agriculture to wastewater treatment. A group of environmental chemists keeps a close eye on long-term effects, exploring ways to trap aluminum from mining runoff or industrial discharges using citrate-based materials. There’s a niche thread of research into its optical and electronic properties, eyeing up uses in specialty coatings or catalysis.
Aluminum compounds have long been in the crosshairs for toxicity research, mostly because aluminum accumulation links to neurodegenerative diseases like Alzheimer’s, though this remains controversial. Studies on aluminum citrate show that, in small controlled doses and through non-oral routes, it presents low acute toxicity. Animal models reveal trouble at higher exposures, particularly for neurological and renal outcomes. Regulators in the US and Europe have outlined acceptable daily intake levels, but these lean heavily toward caution when aluminum citrate is used—especially in supplements or medical settings. Research into its excretion patterns suggests most citrate-bound aluminum leaves the body relatively quickly, yet repeated or high-dose exposure could lead to gradual buildup. Ongoing studies look for better biomarkers to predict risk, focused on sensitive populations like children and those with kidney trouble.
Aluminum citrate won’t disappear from chemistry labs or specialized plants anytime soon. Researchers see potential for scaled-up green synthesis, possibly using bio-based feedstocks or low-energy processes to make manufacturing less wasteful. In environmental science, its future could involve new materials that trap and recycle aluminum from contaminated water streams. Medical research circles around improved formulations that limit toxicity while taking advantage of strong metal-binding. Regulatory bodies will keep watching emerging data, fine-tuning guidelines for use in consumer products. There's a push to understand every angle—biological, ecological, industrial—so future applications rest on a thorough understanding of risks and rewards.
Aluminum citrate turns up in some overlooked places inside healthcare and self-care routines. I’ve seen it listed on some antiperspirant deodorants and topical medications. The science behind this isn’t buried in corporate jargon. Aluminum binds with sweat on the skin’s surface, creating a gel-like plug in sweat ducts. This slows down perspiration. For those living in hot climates or with overactive sweat glands, it’s more than a comfort; it makes daily life manageable. There’s something reassuring about reaching for a product that won’t leave clothes damp right after a morning commute.
Doctors sometimes use aluminum citrate as a phosphate binder, especially in cases where patients have kidney issues and need to keep their phosphorus levels under control. That’s because failing kidneys can’t remove enough phosphorus from the blood. High phosphorus levels can pull calcium out of bones and mess with the body in all kinds of ways—weak bones, itchy skin, heart stress. Binding excess phosphorus so it can leave the body safely isn’t anything flashy, but it’s a life-protective move grounded in decades of medical practice.
Most folks don’t realize aluminum salts, including citrate forms, show up in certain food processing routines. This salt sometimes edges into food as a firming or anti-caking agent. The food industry relies on consistency in baking powders and processed cheese slices, and aluminum compounds keep things from clumping or losing structure. From my own kitchen, I know crumbly, soggy cheese won’t get eaten—function matters more than perfectionism on a nutrition label, though discussion about long-term aluminum intake continues in scientific circles.
Plenty of questions swim around any additive containing metals. Decades back, there were big headlines about links between aluminum and health worries like Alzheimer’s. Most large studies have failed to show clear evidence connecting everyday aluminum exposure to serious disease in healthy people. Regulatory bodies like the FDA and EFSA keep limits in place to prevent chronic high intake, but for most people, everyday products containing small amounts remain within safety guidelines.
I keep an eye on these topics not just for personal health, but because younger people and those with kidney trouble can’t clear aluminum efficiently. Products get safer when we keep these things in mind, instead of letting a few industry voices decide everything.
One way to improve peace of mind and product safety involves clearer labeling, especially for people at risk—patients with compromised kidneys, kids, the elderly. This could push companies and researchers to keep investigating low-dose, long-term exposure and hunt for alternatives where needed. Changing regulations often drags, so public awareness and pressure matter.
I want to see continued investment in studying how aluminum compounds act in real bodies, not just test tubes. Society can’t always rely on old data from the last century. New insights could point toward safer ways to manage sweat, stabilize food, or control blood chemistry in medical settings.
Aluminum citrate might not make the news every day, but it shapes real experiences for countless people—from keeping a hospital patient healthy, to helping a construction worker avoid sweat stains, to making sure food stays fresh. Knowledge and attention from both regulators and users leave a mark on how ingredients like this serve the public good.
Aluminum citrate turns up in some food, drinks, and supplements. The reason for its use comes down to helping with shelf life, texture, and sometimes acting as an acidity regulator. Folks shopping for supplements or even some processed foods might spot it listed among the ingredients. It sounds technical, but for most people, it’s just another long name in fine print. Concerns get more serious when people stop and think about the word "aluminum," especially considering its use in things outside the kitchen, like antiperspirants or water treatment.
Food and health authorities look at additives like aluminum citrate with plenty of skepticism and science behind them. The U.S. Food and Drug Administration recognizes certain aluminum compounds as generally safe when used as intended in regular food amounts. European regulators also track aluminum intake, with warnings about going overboard. Both sides warn about chronic high intake—mainly due to the fact the human body struggles to clear aluminum efficiently.
One thing most doctors and nutritionists agree on: healthy kidneys pull aluminum from the bloodstream and help send it out of the body with little trouble. Most folks eating balanced diets and drinking safe water won’t store much aluminum. For people with weak kidneys, like those with chronic kidney disease, the situation changes. In those cases, the body can’t move the aluminum out fast enough, which sometimes leads to bone or brain problems over time.
Decades back, “dialysis dementia” showed up in hospitals, and the link got traced to aluminum exposure in folks on long-term dialysis. The medical field responded by overhauling how water used in dialysis gets treated. That move brought the number of cases down a lot. For healthy folks, most studies just don’t see much of a problem from everyday exposure—especially at levels found in regular diets.
Still, nobody wants mystery ingredients building up quietly in their system. Excessive aluminum—whether from food additives, cookware, or medications—won’t do the brain, bones, or nerves any favors in the long haul. It’s not just a single food ingredient. Personal care routines, antacids, and heavily processed foods can all add a bit more aluminum to the total. All the little bits add up faster than most people think.
Reading ingredient lists feels tedious until a health scare crops up. People who already need to watch their mineral intake, like older adults or those with kidney troubles, benefit from sticking to straightforward foods without lots of extras. Asking a doctor before taking any supplement with unfamiliar ingredients remains a smart move. Nobody loses by leaning on fruits, vegetables, and whole grains. Most processed choices add more than just flavor.
Regulators keep track of studies and adjust rules when needed. Meanwhile, industry keeps finding new additives and ways to tweak products. Being cautious costs nothing. Ask questions, eat simply, and pay attention to your own health over chasing whatever’s newest on the shelf. Maybe that’s not thrilling. It bets on something tried-and-true: fewer additives mean fewer surprises down the line.
Aluminum citrate finds its way into a lot of products. Some people use it as an ingredient in medicines, while food and drink manufacturers add it to processed goods, and medical labs rely on it for different technical reasons. Yet, like many additives that sneak into modern routines, aluminum citrate does not come without risks.
Most people do not think about where aluminum lands in the body after taking a supplement or eating food with additives. Yet, bodies process aluminum differently. Some reports show that people may end up with constipation after exposure to aluminum compounds. Sometimes, stomach cramps, feeling queasy, or diarrhea can show up too. In rare cases, unusually high use can stress out the digestive system or trigger changes in how the kidneys work.
People who deal with kidney struggles might run into bigger problems. Healthy kidneys pull heavy metals out of the body, but damaged kidneys leave extra aluminum floating around. Over time, this build-up can create serious trouble—think memory loss, bone problems, or muscle weakness. Poison control centers sometimes see these patterns in people who accidentally get too much aluminum citrate, whether through medicine or from not realizing how much has built up across months.
Research in scientific journals warns about the risk of brain-related symptoms. For example, the National Institutes of Health reports that large doses can link to confusion, feeling tired all the time, and nerve symptoms that look a lot like those in dementia. It rings especially true for the elderly, or people with long-term illness.
Doctors sometimes guide people who have kidney issues or who take lots of antacids or phosphate-binding medicines to skip aluminum citrate. The U.S. Food and Drug Administration keeps a close watch on aluminum in products given to newborn babies or folks in intensive care. Infants’ kidneys have not finished developing, which makes them vulnerable to heavy metal overload. Long hospital stays with many intravenous shots can stack up aluminum dose quickly, which the FDA notes as a serious concern for parents and caregivers.
Studies link long-term aluminum exposure to more than just stomach trouble or sluggish thinking. Over years, bones can become fragile and break more easily. People diagnosed with osteoporosis and weak bones share some overlap with those who take lots of aluminum-based medicine. Scientists have also tracked possible ties between high aluminum and diseases like Alzheimer’s, although many experts still debate how strong that link truly stands. One known fact: high aluminum in drinking water or supplements never helps those who already wrestle with chronic disease.
Health experts encourage people to check ingredient lists and choose food or supplements with less processed content. Those with kidney issues should speak regularly with their care team, double-checking any medicine for unwanted aluminum ingredients. Government public health sites, like those from the CDC and NIH, update their guidelines each year. I recommend using those resources to make smart decisions—especially for young families or older relatives. If someone notices a lingering upset stomach, mood changes, or signs of weakness, seeking advice from a healthcare provider always matters.
Aluminum citrate shows up across laboratories and some industrial settings. Storage often flies under the radar, but habits around it leave a major footprint on both safety and product reliability. Many people look at a white powder in a jar and forget that moisture, light, and contamination don’t take breaks. I’ve seen ruined batches and dust leaks because someone thought any cupboard would do. Looking at the risks, a few simple routines help keep issues at bay and reduce waste too.
A lot of reference guides put aluminum citrate under “room temperature, dry place.” Anyone in a real-world lab knows that temperature and humidity fluctuate. An air-conditioned server room means one thing, a satin-glove factory in the rainy season is a different story. Humidity slips through even tight containers, especially when containers are opened and closed. If aluminum citrate gets damp, it clumps, and the chemical profile shifts, opening the door to unpredictable sample quality. Quality counts for more than most folks assume, especially where trace reactivity affects downstream results.
Sharing space with volatile substances does aluminum citrate no favors. Strong acids, solvents, and reactive metals produce corrosive gas or flying particles—unexpected chemistry starts to happen. Keeping this compound on a shelf next to bleach, acetone, or even zinc pushes its stability to the edge. Separate cabinets and using containers made of HDPE or borosilicate glass cut down the odd hazard. From my own routines, I trust tight screw caps over snap-tops. Everything labeled—dates, hazard codes, and who opened it last—adds a layer of accountability.
Sunlight triggers odd reactions, especially over weeks or months. UV-induced breakdown speeds up if water vapor gets in. Freshness and activity slowly fade, and sometimes impurities creep in that change how the compound works. Amber containers or opaque cabinets serve a crucial role. Anyone working through a container over many uses should keep out the habit of leaving lids off during pipetting or scooping. I’ve lost count of times I’ve seen an unsealed sample left on a counter, only for a sticky mess to show up within days.
Long-forgotten containers pile up. Cross-checking expiration dates every quarter helps keep things orderly. Bringing a “first in, first out” routine makes a difference, especially as spoiled or discolored batches should head to hazardous waste. Cutting corners by dumping residues down a drain not only breaks regulations, but also harms the water supply. Labs that keep a central log of chemical inventories almost always avoid last-minute scrambles and surprises.
Good storage habits start at the purchase stage—smaller jars, less risk. Storing aluminum citrate in a cool, dry, well-ventilated cabinet with minimal light exposure avoids most of the usual pitfalls. Regular inventory checks and clear labeling keep everyone safe and the material effective. Small investments in sealed containers and storage space pay off by safeguarding quality, avoiding costly repeat tests, and cutting down emergency clean-ups. Working in labs, I have learned there are fewer headaches sticking to these simple rules than dealing with unexpected spills or spoiled chemicals.
Anyone reading the fine print on a prescription bottle will notice the long list of possible interactions. But some ingredients, like aluminum citrate, don’t get as much attention outside the pharmacy. Most people connect aluminum with soft drink cans or cookware, yet the compound ends up in quite a few antacids and phosphate binders. I remember back in college grabbing antacid tablets from the campus store before finals, never considering how they might mix with the allergy medicine I’d started that spring.
Aluminum citrate works by binding phosphate in the gut, which helps some folks with kidney conditions. But its ability to bind other things turns into a problem for people taking medications by mouth. Antibiotics such as tetracyclines and quinolones get a lot of press in this area. The aluminum forms a complex with those antibiotics, making them much harder for the body to absorb. That means the infection doesn’t get the punch it needs from the medicine, which boosts the risk of resistance or a slow recovery. Sometimes a doctor’s directions sound needlessly fussy: “Take this antibiotic two hours before or after you chew those antacids.” They’re not just being overly careful—timing matters.
It’s not just antibiotics. Thyroid replacement therapies, some heart medications, and even simple vitamins like iron can get caught up in aluminum’s binding effect. Thousands of patients in hospitals end up with odd spikes and dips in their thyroid hormone levels, only to find out later that a common phosphate binder was blocking their absorption. It’s the kind of thing that slips under the radar, especially for people who pick up supplements and over-the-counter remedies without chatting with a pharmacist.
Even without prescription drugs in the mix, the body can hang on to more aluminum than it should if the kidneys aren’t filtering well. This makes those with chronic kidney disease more vulnerable. Once, I saw a family friend suffer memory issues that the doctors first chalked up to aging, until a medication review revealed her kidney disease didn’t mix well with her aluminum-containing antacid. That case taught me to never brush off those weekly medication reviews pharmacies offer now—sometimes small adjustments have big payoffs.
Tackling this problem starts with clear conversations. Patients can bring a printed list of all medicines and supplements during any doctor’s visit, which helps catch these mix-ups early. Pharmacists now have sophisticated computer systems that flag these risky pairs, and that helps avoid trouble. Still, the challenge remains because not every health system connects the dots between hospital and community pharmacy lists.
Some experts urge companies to label their non-prescription products more clearly about interaction risks, putting plain-language warnings on bottles of antacids using aluminum citrate. That could help, but habits play a bigger part. A good habit is to ask a healthcare professional before starting new supplements or remedies, not waiting until side effects show up. People with complicated medication regimens or kidney disease need extra vigilance. Even if it means carrying an updated medication list on your phone or wallet card, the effort pays off.
There’s no substitute for transparent advice from trusted professionals. Aluminum citrate isn’t in the headlines every day, but its impact touches people who just want relief from heartburn, indigestion, or phosphate imbalances. Small conversations between patients and healthcare teams make all the difference in keeping everyone safe from unexpected medication interactions.
| Names | |
| Preferred IUPAC name | aluminum 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Citric acid, aluminum salt Aluminium(III) citrate Aluminum tris(citrate) Aluminum citrate |
| Pronunciation | /əˈluːmɪnəm ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 31142-56-0 |
| 3D model (JSmol) | 3D model (JSmol) string for Aluminum Citrate: ``` Al3(C6H5O7)2 ``` |
| Beilstein Reference | 3588564 |
| ChEBI | CHEBI:29967 |
| ChEMBL | CHEMBL4297856 |
| ChemSpider | 13094 |
| DrugBank | DB11289 |
| ECHA InfoCard | ECHA InfoCard: 100.033.952 |
| EC Number | 241-487-1 |
| Gmelin Reference | 16418 |
| KEGG | C18742 |
| MeSH | D000698 |
| PubChem CID | 159404 |
| RTECS number | KH5550000 |
| UNII | 4H77HBB9U9 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID7053992 |
| Properties | |
| Chemical formula | Al₇(C₆H₅O₇)₆ |
| Molar mass | 594.13 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 2.47 g/cm³ |
| Solubility in water | Very soluble |
| log P | -10.6 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.5 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | -23.1×10⁻⁶ cm³/mol |
| Viscosity | Viscous liquid |
| Dipole moment | 2.62 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 367.6 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | A02AB05 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. May cause skin irritation. |
| GHS labelling | GHS labelling of Aluminum Citrate: `"Warning; H319: Causes serious eye irritation; P264, P280, P305+P351+P338, P337+P313"` |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | P264, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | Oral rat LD50: 15.9 g/kg |
| LD50 (median dose) | LD50 (median dose): 282 mg/kg (rat, oral) |
| NIOSH | KWQ134 |
| PEL (Permissible) | 15 mg(Al)/m3 |
| REL (Recommended) | 160 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Aluminum sulfate Sodium citrate Potassium alum Aluminum chloride Aluminum acetate |