Curiosity has driven chemists for centuries, and ammonium bismuth citrate carries that spirit in its roots. Back in the 19th century, as analytical techniques blossomed, researchers began uncovering the nuances of metal citrates. Bismuth compounds, with their heavy atoms and distinctive properties, attracted attention in pharmacies and material science labs. People started blending bismuth with organic acids like citric acid, and the addition of an ammonium group opened new doors for solubility and formulation. By the early 20th century, ammonium bismuth citrate had cropped up in research papers and reference books, often alongside efforts to improve imaging for medical diagnostics and fine-tune chemical reactions in glassmaking. Its role expanded as laboratories learned to isolate it reliably, proving useful for both industry and research.
Ammonium bismuth citrate puts together bismuth, ammonium, and citric acid into a pale yellow or sometimes off-white crystalline powder. Companies supply it in tightly sealed containers, keeping out moisture because exposure can mess up its stability and reactivity. Manufacturers ship it as a reagent grade chemical for labs, but it’s also available in bulk for industry. Its most welcome application often lies in medical imaging, since it offers contrast properties that help make X-rays clearer. Quality control in production looks for purity and accurate proportioning of the three foundational parts — bismuth, ammonium, and citrate — to guarantee users get the expected outcome.
This compound stands out for its solubility in water, setting it apart from other bismuth salts that remain stubbornly insoluble. Its color makes visual checks possible, while its fine texture lets it dissolve swiftly. Ammonium bismuth citrate handles heat up to moderate levels, but strong acids and bases break it down. Chemists respect bismuth’s relatively low toxicity, another reason this citrate salt gets picked for sensitive scientific processes. On a chemical level, the bismuth acts as a central ion, with citrate bringing in those oxygen-rich arms for chelation, and ammonium balancing everything on the outside. Some labs measure its pH stability to dial in conditions for experiments or manufacturing recipes.
Every package should carry clear details. Manufacturers list purity, moisture limits, and assay values, usually expressed as bismuth content by percentage. The label will show molecular formula and weight, hazard statements in line with global standards, directions for storage, and batch numbers to help trace the chemical’s path back to the source. Labs check for contaminants like lead, cadmium, and other heavy metals, since the presence of these can compromise applications in medical or food-related items. Anyone handling the material finds hazard pictograms pointing out things like irritation potential or environmental precautions.
Crafting ammonium bismuth citrate isn’t flashy but rewards careful hands. Chemists take bismuth nitrate and react it with citric acid in water under gentle heating. Ammonia solution comes next, coaxing the three components to join in a single salt. Filtration, concentration, and slow evaporation coax out clean crystals. Yields and purity improve when the operator watches temperatures and tweaks the pH just right. Some plants run large batches, envying the repeatability found in a well-tuned synthesis line. Proper rinsing strips out unreacted ammonia and acid, delivering a product ready for analysis or next-step processing.
Once in hand, ammonium bismuth citrate serves as a springboard for other bismuth compounds. Heating it coaxs out oxides, which find use in glass coloring and ceramics. Adding more acid swaps out the ammonium for hydrogen, leading to bismuth citrate itself. Some researchers experiment by tossing in organic amines or replacing ammonium with other cations, trying to tune physical properties or solubility for new products. In the right conditions, the citrate arm can break down, freeing bismuth for catalytic work or as a component in luminous materials. These modifications don’t just stop at the bench — some scale up for industrial batch runs.
Chemists know this compound by many other names. Sometimes they call it bismuth ammonium citrate, or use systematic variations like ammonium bis(citratobismuthate(III)). Labels in catalogues might carry supplier-specific names, or even abbreviations tied to product codes. Improper naming can introduce confusion — bismuth citrate isn’t the same thing, for example, and the ammonium makes a difference in how it behaves. Regulatory paperwork or customs use these synonyms, so anyone ordering or declaring shipments double-checks their documents to avoid mistakes or shipment delays.
Working with ammonium bismuth citrate calls for routine safety precautions: gloves, goggles, and good ventilation. While bismuth ranks among the less toxic heavy metals, inhalation or swallowing can bring on discomfort or organ irritation. Lab workers make sure dust doesn’t float into the air, and weigh quantities with care to avoid small spills. Emergency showers and eyewash stations back up those who handle larger batches, and spills need tidy cleanup with minimal dust dispersal. Disposal runs through hazardous waste channels, given the presence of both bismuth and ammonium ions. Companies keep records to prove proper handling and limit exposure risks for both workers and the wider community.
Radiology and photography labs lean on ammonium bismuth citrate as a contrast agent, boosting the clarity of certain images. The ceramics industry uses it for glazes and colors that hold their intensity under high heat. Water treatment and catalysis also call for this compound, tapping into bismuth’s affinity for certain contaminants or its ability to steer chemical reactions. Some research groups see value in its antimicrobial traits, experimenting with coatings and mixtures for medical devices. In some countries, regulators ask for purity guarantees before allowing these uses, especially in products destined for patient or consumer contact.
Ongoing research chases new uses and improvements in formulation. Teams push beyond contrast imaging, probing whether tailored forms of ammonium bismuth citrate can knock out bacteria or become safer alternatives in electronics manufacturing. The interplay between bismuth, ammonium, and citrate attracts computational chemists mapping out reaction mechanisms. Each new publication tweaks ideas about the structure or opens up possible chemical derivatives for industry. Toxicity studies run side by side with these projects since safety remains central to scaling up any novel use. Engineers look for ways to boost yields, cut waste, and lower energy use during synthesis.
Medical uses mean toxicity gets plenty of scrutiny. Bismuth salts can trigger kidney problems at high doses, which puts a ceiling on how much gets used in pharmaceutics. Scientists test how the combination with citrate and ammonium impacts absorption and elimination from the body. Animal studies, cell cultures, and in-vitro systems sort out whether ammonium bismuth citrate breaks down into simpler — and possibly more harmful — parts. Regulators respond to these findings by setting exposure limits and outlining necessary safety measures on labels and handling guides. Researchers keep looking for ways to drop toxicity, whether by tweaking the formula or shifting to more stable compounds under real-world use.
Green chemistry and sustainability steer the roadmap for ammonium bismuth citrate’s future. Heavy metals draw skepticism from regulators, but bismuth’s softer toxicity profile keeps it in the running for sensitive roles. Demand grows for imaging agents that offer clarity without raising environmental worries. Electronics manufacturers hunt for bismuth-based alternatives to more hazardous metals, focusing on recyclability and safe disposal. Research labs compete to discover tweaks to the core structure, nudging its physical and chemical traits toward new frontiers in healthcare, environmental engineering, and nanotechnology. Everybody from chemists to policymakers watches these developments, knowing that safety, utility, and cost will shape where ammonium bismuth citrate lands in the coming years.
Walk into a medical lab or a factory making everything from cough syrups to hair dye, and odds are something like ammonium bismuth citrate plays a background role. This compound doesn’t make headlines, but its applications run wide. I remember chatting with a pharmacist friend about how certain ingredients in health products barely get a mention, yet the difference they make to quality or consistency is huge. Ammonium bismuth citrate is one of those unsung helpers.
In the medical field, diagnostic imaging often relies on bismuth compounds. Ammonium bismuth citrate steps into the spotlight in barium meal alternatives and related products, helping doctors see inside the body with greater clarity. Some research papers highlight how the compound’s chemical stability and tolerance for mildly acidic environments allow excellent contrast in X-ray examinations. Patients who have trouble with traditional barium mixes benefit from these alternatives, which can be gentler on the stomach.
Head over to the cosmetics shelf and, tucked behind vibrant labels, you’ll find a few skin ointments and hair care products that count on this substance. It brings value in hair dyes and colors because of its ability to interact predictably with other ingredients, giving richer, more lasting shades. The bismuth element, recognized for its low toxicity compared to heavy metals, helps formulate products that are a bit safer for repeat use, particularly in personal care.
Curiously, the food industry finds selective uses too. The compound’s mild antimicrobial features make it part of select additives for preserving freshness in prepared foods. Even though bismuth salts aren’t as common as sodium-based preservatives, some companies experiment with compounds like ammonium bismuth citrate given their safety profile in regulated amounts. Having processed food at home during the pandemic, food preservation’s small tricks and additives stood out to me—each one matters when shelf life and safety come into play.
In manufacturing, the chemical pulls its weight as a catalyst. Paints, ceramics, and some specialized glass products benefit from controlled reactions and color stability thanks to this compound. Companies concerned with producing stable, high-quality pigments often explore bismuth-based agents to meet the demand for safer, less toxic alternatives compared to older heavy metal formulas.
It’s easy to overlook questions about safety with obscure chemicals. Regulatory bodies like the FDA in the US have set standards for the use of bismuth salts, including ammonium bismuth citrate, in over-the-counter products. Experts have pointed out that, compared to substances like lead or cadmium, bismuth compounds rarely accumulate in the body and pose less risk in everyday exposure. Having seen several reports from medical and environmental safety agencies, I understand why safety thresholds and regular reviews stay important—too much of any compound can tip the scale towards risk.
Moving forward, more researchers are picking apart the fine details of how these compounds interact with living systems and the environment. Calls for cleaner manufacturing and reduced chemical waste push companies to rethink ingredients, track their environmental impact, and look for alternatives where possible. Studies explore biodegradable carrier systems for bismuth compounds, aiming for the same results with fewer unintended consequences.
The story of ammonium bismuth citrate follows a familiar trail: a quiet helper that keeps some of our daily comforts running smoothly, while raising important questions about safety, regulation, and finding a better chemical balance in the future.
People often hear about chemical additives and feel a wave of concern. Ammonium bismuth citrate shows up in some diagnostic medical products and a handful of pharmaceutical uses. It comes from bismuth and forms part of a larger family of bismuth compounds, many of which have made their way into medicine cabinets over the years. Think of bismuth subsalicylate—that chalky liquid for upset stomachs—which has a long record of safe use with proper dosing.
Regulatory bodies take a keen look at the safety of compounds that wind up in food or medicine. The Joint FAO/WHO Expert Committee on Food Additives has set strict limits on many bismuth derivatives. Studies show bismuth itself can help treat digestive problems, but too much can pose a real risk. The citrate form, paired with a bit of ammonium, helps with solubility and absorption for specific diagnostic uses. Medical research finds that, in controlled settings, small doses rarely cause trouble for most healthy adults.
Reports in the medical literature rarely mention ammonium bismuth citrate outside of hospital or clinic settings. Most bismuth toxicity cases go back to chronic use or overdosing—not brief exposures. Symptoms often pop up only after heavy, ongoing intake over weeks or months, leading to issues like kidney strain or neurological problems. Kids, pregnant women, or patients with kidney trouble can face higher risks.
Consumers put faith in the doctors and regulators watching over product safety, but not every country holds these ingredients to the same rules. Here in the United States, the FDA hasn’t widely approved ammonium bismuth citrate for food or regular over-the-counter use. If a product says it contains this compound, it’s not a regular pantry item—chances are it’s part of a medical test or specialty prescription.
Transparency makes a real difference. Honest labeling, with side effect warnings and clear guidelines, helps protect everyone. In countries where it does show up as an ingredient, even in trace amounts, watchdog groups and academics keep a close eye on new research.
It pays to read ingredient labels and talk with your doctor before trying anything unfamiliar—especially if it sounds like a chemistry class flashback. No one should self-medicate with chemical powders or tablets just because someone online claims they’re safe. The body handles small amounts of bismuth pretty well, but adding ammonia and citrate changes the equation. Without proper dosing, side effect risks tick up. Long-term safety data still lies mostly in the hands of specialty doctors and not everyday consumers.
For the most part, anyone with kidney disease or a sensitive stomach should steer clear, unless a doctor gives the green light. Before regulators allow widespread use of any compound, they demand thorough toxicology studies, a clear understanding of how the body processes the chemical, and real-world safety data—not just lab results.
Researchers and regulators need to collect more evidence on long-term exposure and real-life use, not just tightly controlled studies. Doctors and pharmacists should remain vigilant and record any side effects spotted in their patients. Above all, manufacturers and advertisers need to keep claims honest and stick to data-backed statements. Until clearer information arrives, ammonium bismuth citrate should stay in specialized settings, away from home kitchens and unsupervised medicine cabinets.
Growing up in a family that ran a neighborhood hardware shop, I picked up a lesson early: mess around with chemicals or heavy-duty cleaners, ignore the labels and guidelines, and sooner or later, something nasty comes your way. Ammonium Bismuth Citrate does not draw much attention outside certain labs or industrial settings, but the same mindset applies. Mishandling compounds can lead to safety risks nobody wants, especially where people rely on safe workspaces.
At its core, Ammonium Bismuth Citrate holds together pretty well. It does not toss up sudden surprises if people store it right, but comfort can fool folks. Most suppliers will send this compound in tight, sealed containers—plastic or poly-lined—that shield it from moisture. The smallest sign of water can slowly break it down or, worse, trigger shifts in structure that reduce its utility. There is a science reason behind this, but as a non-chemist with a stake in not wasting money or time, the lesson is simple: keep it bone-dry.
The habit to toss chemicals onto just any shelf—sometimes next to a window, sometimes near a noisy heater—runs deep. Ammonium Bismuth Citrate does not do well if temperatures swing wildly. Direct sunlight speeds up changes or clumping that ruin a batch, and heat vents can push things even faster. Based on personal work at a municipal supply yard, temperature control topped our list. “Room temperature” really means out of direct sun, out of the line of space heaters, and well away from sources of moisture. Stores aiming for a long shelf life invest in clean, shaded, and dry rooms that stay in the 20–25°C (68–77°F) zone. That’s what keeps the stuff usable into next season.
Labels save lives and reputations. I’ve seen coworkers snatch the wrong bottle and trigger chaos, simply because faded labels left too many guesses. For Ammonium Bismuth Citrate, clear labeling keeps confusion low. Date the container, mark the contents, and warn against mixing or eating. In places where language can cause mix-ups, pictograms add an extra layer of safety. Older hands in the lab train anyone new on these rules, because a mix-up can waste a day’s worth of work or worse.
Mixing chemicals in tight quarters creates the biggest headaches. Separate storage of reactive materials isn’t just a box-ticking exercise. Ammonium Bismuth Citrate, while not explosive or highly flammable, can react in unpredictable ways with acids or oxidizers. One spill in the wrong spot, and a benign powder turns into a disposal or health headache. Even in places where space runs tight, keeping separators—simple plastic bins or trays—adds an extra barrier. I’ve learned to never take shortcuts on this front.
Old stock builds up because people forget about it or assume chemicals last forever. In practice, Ammonium Bismuth Citrate, like many other compounds, starts to drop in quality over time. Improperly stored containers clump; some spoil to the point of uselessness. A simple routine using first-in, first-out tracking helps. Disposal follows local waste protocols, never just down the drain—protecting both workers and the local environment.
Chemical storage does not improve through fancy technology alone. Clear labeling, moisture control, steady temperatures, and strict separation prevent most mishaps. Investing in ongoing staff training stops forgetfulness from snowballing into a mess. Teams that check containers regularly catch issues faster. By sticking to proven routines, labs and stores keep Ammonium Bismuth Citrate—among other things—from turning into a health risk or financial loss.
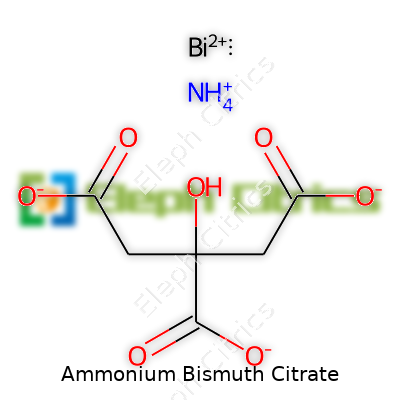
Chemistry classes hand formulas to students by the dozen, but each one tells a story about real-world use. Ammonium Bismuth Citrate is a compound with both industrial and medical ties. Its chemical formula goes as (NH₄)₄[Bi(C₆H₅O₇)₂]·2H₂O, which lays out all its elements: the ammonium ion, bismuth, citric acid, and two water molecules thrown in.
This compound isn’t something you find on a grocery store shelf, but it shows up in more lives than most folks expect. On the medical side, bismuth compounds help fight stomach issues like ulcers and even Helicobacter pylori bacteria. Some radiology procedures lean on it for contrast during imaging tests. The reason? Bismuth’s heavy atomic weight shows up clearly, and the ammonium citrate part keeps it soluble. Its presence matters because it offers a safer profile than some other metals, leading to fewer side effects for patients who already feel rough.
The formula (NH₄)₄[Bi(C₆H₅O₇)₂]·2H₂O spells out the sum of all its players. Ammonium brings four nitrogen atoms alongside hydrogen, all there to balance out bismuth’s charge among the two citrate molecules. The citric acid part—C₆H₅O₇—binds the metal securely. Water of crystallization rounds it out, keeping the structure stable and ready for transport or mixing into formulations. That water isn’t just dead weight; crystallized water can mean longer shelf life, easier dosing, and simpler preparation in a lab or pharmacy.
There’s a big difference between blurring through formulas and really seeing what they mean. Quick mistakes in something as simple as a chemical equation can lead to batch recalls or worse when human health enters the picture. Pharmacists trust these numbers to keep dosages safe. Scientists depend on them for predictions. In university research, I once saw an entire trial fail just because someone swapped in a similar-sounding compound by accident. Getting the formula right from the start means no crossed wires later.
Mistakes in chemistry can feel abstract until a wrong formula makes a drug less effective or flat-out dangerous. Students mostly memorize formulas for the test, but the moment that knowledge meets the real world—labs, hospitals, factories—the risk jumps. The best defense is double-checking: updated safety sheets, peer review in labs, and digital tools that highlight errors. Teaching should root formulas in context, not just rote learning. Building skills for cross-checking work lets professionals catch those mistakes early, saving money and protecting people at the same time.
Once you’ve seen how formulas set the stage for healthy outcomes, trust in chemistry steps up a notch. Ammonium Bismuth Citrate stands as a solid choice for patient care and diagnostics, but only if every chemist, technician, and pharmacist approaches it with an eye for accuracy and strong science.
Ammonium Bismuth Citrate doesn’t sound like a household name, but it shows up in labs, research centers, and sometimes in manufacturing plants. Few people outside those fields know much about it. You spill this stuff on the ground, and suddenly you’ve got a situation on your hands. I’ve worked in a lab where chemical spills weren’t exactly rare, so I know from experience that even so-called less hazardous chemicals demand respect. That white or yellowish powder starts to dust up, and you don’t want to be breathing it in or getting it on your skin.
Even if a material has low acute toxicity, repeated exposure can hurt people, especially if safety steps get skipped. Nobody wants rashes, eye irritation, or worse, upset stomachs from accidental ingestion. I’ve seen coworkers get comfortable just brushing off powders with shop towels, but that never ends well. Bismuth compounds carry risks, and ammonium ingredients have their own hazards. Acting fast and smart protects not only the person on cleanup duty but everyone else in the lab or building.
If a spill happens, don’t go near it without glove protection and goggles. Respirators or at least well-fitted masks can make a difference, especially in closed rooms with poor airflow. In my early years, I watched a technician cough for an hour after sweeping up a fine powder with no mask. That lesson stuck with me. Long-sleeved lab coats keep the dust off your arms. Closed shoes are a must—open toes and chemicals never mix. If you can, tie your hair back so you’re not brushing it later and shaking out particles.
It’s tempting to just wipe up powder and hurry along, but dry material will go airborne if you move too quickly or sweep aggressively. Dampen the area with a little water or proper spill control solution to reduce dust. Scoop up material with rigid plastic tools, not your hands. Dispose of everything—tools, gloves, used rags—according to your facility’s hazardous waste rules. Dumping things in general trash isn’t just unsafe, it might land you in trouble with environmental regulators. I’ve seen labs save a headache by having quick-access chemical waste bins right where work happens.
Once the loose powder is up, wash that area with soap and water for good measure. Nobody wants to leave invisible traces behind. Dry the surface thoroughly. I have noticed some people forget to check if powder got tracked out on shoes. Place a wet towel or sticky mat at the door to catch leftovers. A regular review of spill procedures and practicing a dry run with your team builds confidence—people won’t freeze if an accident happens for real.
Most spills can be handled safely with prep, common sense, and by not cutting corners. These days, I check my safety kit before each shift—fresh gloves, ready-to-go spill pads, and a marked container for the waste. Facilities should put up clear signs, keep emergency numbers visible, and keep the Material Safety Data Sheet easy to find. These steps don’t take much time, but they can mean the difference between a quick fix and a bigger problem.
| Names | |
| Preferred IUPAC name | Ammonium 2-hydroxypropane-1,2,3-tricarboxylate;bismuth(3+);dihydrate |
| Other names |
Ammonium bismuthyl citrate Bismuth ammonium citrate |
| Pronunciation | /əˈmoʊniəm ˈbɪzməθ ˈsɪtrət/ |
| Identifiers | |
| CAS Number | 61329-23-1 |
| Beilstein Reference | 13210453 |
| ChEBI | CHEBI:85258 |
| ChEMBL | CHEMBL2106031 |
| ChemSpider | 21768267 |
| DrugBank | DB11150 |
| ECHA InfoCard | 07c6f1f1-69cd-4ee9-92ae-b88f8e41d56d |
| EC Number | 425-720-9 |
| Gmelin Reference | 85241 |
| KEGG | C18640 |
| MeSH | D000648 |
| PubChem CID | 21724738 |
| RTECS number | CU8220000 |
| UNII | 0M2SRZ8ABK |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID9037124 |
| Properties | |
| Chemical formula | (NH4)6[Bi2(C6H4O7)4] |
| Molar mass | 857.21 g/mol |
| Appearance | Light yellow powder |
| Odor | Odorless |
| Density | 2.6 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -4.3 |
| Acidity (pKa) | 5.3 |
| Basicity (pKb) | 7.58 |
| Magnetic susceptibility (χ) | -77.0e-6 cm^3/mol |
| Dipole moment | 0 D |
| Pharmacology | |
| ATC code | A02BX05 |
| Hazards | |
| Main hazards | May cause respiratory and skin irritation. |
| GHS labelling | GHS07, Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319, H335 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| LD50 (median dose) | LD50 (median dose) for Ammonium Bismuth Citrate: Oral, rat: >2,000 mg/kg |
| NIOSH | WA2625000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Ammonium Bismuth Citrate: Not established |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | Not listed. |
| Related compounds | |
| Related compounds |
Bismuth subcitrate Bismuth subsalicylate Bismuth citrate Ammonium citrate Bismuth nitrate |