Ammonium citrate enters the chemical history books through a thread of agricultural and medical uses dating back to the nineteenth century. Chemists such as Justus von Liebig studied citric acid’s effects on soil nutrition, but only after the rise of industrial chemistry did ammonium citrate step out of the lab and into commercial production. Early researchers experimented with mixing ammonium salts with citric acid to produce a compound useful not just for laboratory analysis, but for fertilizer blends. Large-scale industrialization in the twentieth century meant ammonium citrate showed up in food preservation studies, textile processing, and testing for metals, especially iron and aluminum. Despite shifting focus among new synthetic compounds, knowledge gathered from these practical trials still anchors ammonium citrate in both historical chemistry and industrial growth.
Ammonium citrate appears as a colorless, crystalline solid or powder with a mildly tart taste, echoing the flavor profile of citric acid. Whether supplied as mono- or diammonium salts, its main use sits in fertilization and laboratory analysis. Food-grade ammonium citrate plays a role in regulating acidity and helps with dairy product stabilization. Its stable, water-soluble nature simplifies its use across multiple sectors: from buffer agent in research labs, to a leavening ingredient in baking, to a chelating agent that binds to various metal ions. Bulk suppliers categorize products by ammonium salt content and citric acid origin—sometimes from corn, sometimes from sugar beets or cane.
The physical aspect shows itself right away: a white, crystalline structure that dissolves easily in water, giving off no odor. Molecular weights differ based on the degree of ammonium substitution: diammonium citrate holds the formula (NH4)2C6H6O7, with a molecular weight close to 243.13 g/mol. Melting points sit above 170°C, but heat causes gradual decomposition before any rapid melting happens. It reacts slightly acidic, with a pH between 5 and 7 for a standard solution. Technicians can depend on its high solubility (greater than 50 g/100 mL in water at 20°C), a property that lends itself to rapid dissolution for homogeneous mixtures.
Technical-grade products list ammonium citrate content, purity percentages, and trace impurity limits. Food-grade materials require more detailed labeling, calling attention to source, allergen potential, permitted additives, and batch numbers for traceability. Certificate of Analysis documents often accompany shipments, listing appearance, pH, solubility, and heavy metal thresholds. In the laboratory, bottles bear pictograms under the Globally Harmonized System, alerting to irritation or reactivity risks. Understanding these specifications avoids uncertainty in both scientific and industrial settings.
The most common preparation method involves the neutralization of citric acid with aqueous ammonia. Producers first dissolve citric acid in distilled water, then add ammonia slowly with continuous stirring, measuring pH at each step. Complete reaction yields diammonium citrate or, with less ammonia, the monoammonium salt. Solids crystallize out upon cooling or through evaporation, and repeated washing with cooled ethanol purifies the product. Different ratios of ammonia adjust the final salt composition and determine the intended application, whether analytical, food, or agricultural.
Ammonium citrate doesn’t just sit on the shelf—it joins in where ion exchange and chelation are needed. The citrate ion’s three carboxyl groups bind strongly to metallic cations, making it a top choice for dissolving metals in laboratory tests. Chemists can swap out the ammonium ion for sodium or potassium, making closely related salts with different solubility and reactivity. Heating ammonium citrate, especially under dry conditions, leads to its breakdown releasing ammonia, water, and a mix of citric acid breakdown products such as aconitic acid. This property gets harnessed in controlled-release fertilizer formulations and in experiments studying nitrogen loss from soil amendments.
Ammonium citrate wears several names depending on audience and region. Industry catalogs often call it diammonium citrate, monoammonium citrate, or simply ammonium salt of citric acid. Chemical supply houses might list E380 (according to EU food additive codes) or use names like Citrate d’ammonium. Scientists may abbreviate as DAC or MAC to distinguish between substitution levels. Whether in commodity trade or academic research, knowing these aliases cuts through confusion.
Attention to safety never takes a back seat with ammonium citrate. Inhalation of fine dust can cause respiratory tract irritation, and ingestion in high amounts irritates the gastrointestinal tract. Direct skin and eye contact can trigger mild irritation. Operators follow safety datasheets and use gloves, eye protection, and dust control measures in manufacturing and laboratory environments. Facilities maintain proper storage—dry, cool, and well-ventilated conditions keep ammonium citrate stable for extended periods. For spill management, large quantities require containment and careful neutralization, while small spills clean up with water and soap. Compliance with REACH and OSHA guidelines supports safe handling and shipping worldwide.
Ammonium citrate stretches further than fertilizer. In agriculture, it boosts plant growth by providing nitrogen and acidifying alkaline soils. The food industry selects it for baking powder blends and as an acidity regulator in beverages and jams. Textile and tanning industries depend on its ability to sequester heavy metals, improving dye take-up and leather softness. Analytical chemists rely on ammonium citrate as a complexing agent during metal ion titrations and for separating rare earth elements. Water treatment plants sometimes use it to remove unwanted minerals and soften municipal water supplies. Pharmaceutical researchers incorporate it as both a buffer and a dispersing agent in oral suspensions.
Academic and corporate labs keep pushing the limits of ammonium citrate’s versatility. One area of exploration is its use as a green chelate for rare earth metal recovery—a process that cleans up mining waste and recycles valuable resources. Soil scientists measure its effectiveness in slow-release nitrogen fertilizers that aim to reduce nitrate leaching and greenhouse gas emissions in agriculture. New composite materials, including bio-based polymers and hydrogels, tap into ammonium citrate’s promise as a natural crosslinker, increasing water retention and stability. Biomedical engineers investigate how ammonium citrate-based carriers can transport drugs or nutrients within living systems, banking on its biocompatibility and low toxicity. Each project adds layers to its story, making clear that old molecules can learn new tricks in the hands of skilled innovators.
Toxicologists have examined ammonium citrate’s effects through animal studies and occupational exposure monitoring. Low-dose exposure doesn’t show persistent toxicity in mammals, and the compound breaks down into ammonia and citric acid, both readily metabolized by most organisms. High-dose ingestion can cause metabolic alkalosis and nervous system symptoms because of ammonia, especially in individuals with compromised liver function. Workers exposed to dusts report minor irritation, but proper controls minimize risk. Regulatory agencies keep limits on food additive levels and maximum contaminant thresholds to protect public health. Environmental tests suggest that moderate spills degrade quickly in soil and water systems, but ecosystem monitoring remains prudent.
Ammonium citrate stands poised to prove its worth well into this century. Innovators plan to capitalize on its natural origin and biodegradable nature in green chemistry. Wider adoption in slow-release agricultural products can help tackle pollution from traditional fertilizers. As a food additive, it offers a safer alternative in gluten-free and vegan foods where traditional preservation falls short. In the laboratory, increased demand for low-environmental-impact reagents puts ammonium citrate back in the limelight, especially as researchers pivot toward sustainability. Emerging applications in metal recovery hint at untapped value for e-waste recycling and battery technology. With ongoing research and responsive regulation, ammonium citrate joins the short list of legacy chemicals ready for a new wave of responsible industry.
Walking through a science supply store in college, I came across a bottle labeled “Ammonium Citrate.” Only later did I discover all the roles this compound plays outside a chemistry class. Ammonium citrate pops up in food processing plants, metal cleaning factories, labs, and photography studios. Some might pass by its name, but for many industries, this compound keeps things running smoothly.
For anyone reading nutritional labels, ammonium citrate sometimes appears on ingredient lists as a food additive. Food producers use it to adjust acidity, especially in jams, jellies, and drinks. Each batch’s tartness or sweetness can shift, and this compound helps them hit that reliable taste. Ammonium citrate also acts as a chelating agent—it binds to minerals, keeping them from causing trouble in food textures or colors. The U.S. Food and Drug Administration recognizes certain forms as safe for use in food. That transparency gives a sense of security for both producers and shoppers at the grocery store.
Metalworkers and cleaners know ammonium citrate for its role in cleaning and conservation. In my college’s sculpture studio, metal objects tarnished over time. Lab staff showed a trick: make a light solution of ammonium citrate, let the metal soak, and stains dissolve without harsh acids. Museums rely on the same principle but use it to clean old coins, artifacts, and delicate historical objects. By binding with harmful residues, ammonium citrate allows gentle restoration and helps preserve history without damaging underlying materials.
Many lab professionals trust ammonium citrate for its role as a buffering agent and a reagent. I remember mixing solutions for a plant biology lab, measuring the growth response of different nutrients. Ammonium citrate supplied both a nitrogen source for the plants and balanced the pH in the water. In medical labs, it helps stabilize blood samples and acts as an anticoagulant under certain conditions, keeping sample results accurate.
Before the smartphone era, developing photographs involved careful chemistry. Ammonium citrate played a part in making blueprints through a process called cyanotype. Artists and engineers alike, from Anna Atkins (the first female photographer) to industrial designers, have relied on this compound. Its steady performance means clear, detailed prints.
No chemical comes without safety concerns. Ammonium citrate in moderate amounts, particularly in food, is safe. Direct skin contact or breathing in powder, however, can irritate sensitive people. Clear labeling, wearing gloves, and ensuring good ventilation protect those handling it. Public trust grows when companies make information about handling and disposal easy to find. Encouraging sustainable practices means less chemical waste trickling into water systems. Some eco-friendly cleaning brands turn to ammonium citrate instead of harsher acids for exactly this reason.
Ammonium citrate might not steal headlines, but it gets the job done across several fields. Honest information, responsible use, and continuing research can keep this compound both useful and safe. By sharing knowledge about common chemicals like this one, everyone from food producers to artists can make choices that work for people and the planet.
Ammonium citrate pops up in labs, classrooms, and some industrial spaces. It takes the form of a white, granular powder, dissolving easily in water. Many chemists remember mixing ammonium citrate in high school, often while testing for ions or separating metals. Over the years, it’s shown up in photography, cleaning products, fertilizers, and even as a food additive in some limited places. With such broad use, the conversation about its safety keeps rolling on.
Ask any chemist or lab worker and they’ll tell you that handling ammonium citrate with bare hands feels harmless. It doesn't have an aggressive odor. It rarely causes visible skin irritation, unless you’re somebody whose skin reacts easily. Inhalation isn't pleasant—dust can irritate your nose and throat, just as any powder would. I’ve accidentally kicked up enough to get a coughing fit but nothing that left a lasting impact. Yet, nobody wants to eat or breathe unnecessary chemicals, so caution matters.
Regulatory agencies, including the U.S. Food and Drug Administration (FDA), have looked at ammonium citrate in food and medicine. The FDA allows small quantities in certain foods. European authorities also say it’s not a problem in the amounts usually present in fertilizers or products made for public use. The chemical breaks down pretty fast in the environment, not lingering for years as some industrial nasties do.
There’s a solid chunk of research on its impact. Studies back up the claim that it carries mild toxicity, mostly hitting the stomach or skin after big exposures. Large doses can trigger nausea or gastrointestinal discomfort. Gulping large, unregulated amounts could throw off your body’s acid-base balance, but you’d have to go way beyond what anyone would encounter day-to-day.
Long-term, low-level exposure hasn't shown major health effects in workers or lab users. Industrial safety data sheets lay out specific hazards: get it in your eyes and you’ll feel the burn. Swallowing a spoonful isn’t deadly but will leave you feeling miserable. People mixing it every day use gloves and eye protection as you would with any laboratory salt—not because ammonium citrate is especially dangerous, just because it's sensible.
The real issue shows up in workplaces and classrooms lacking clear safety training. Having spent years in educational labs, I saw students ignoring gloves or hurrying through cleanup. This sort of carelessness turns safe substances into hazards. Any chemical handled carelessly, scooped messily, or stored in weak containers can become a problem—think of spillages and cross-contamination.
Safety can’t rely on wishful thinking. Label your containers, follow handling instructions, use PPE—personal protective equipment—when working with powders or liquids, and never take food into the lab. Proper ventilation removes accidental dust from the air. Individuals with asthma or sensitive lungs should alert teachers or supervisors.
There’s always space for improvement. Institutions can offer regular safety refreshers. Teachers should enforce protective gear use, and manufacturers can print safety instructions right on packaging instead of hiding them in tiny manuals. Most accidental exposures disappear with a rinse or drink of water, but any strange symptoms deserve an expert’s attention.
Ammonium citrate doesn’t demand alarm bells, but it calls for steady respect. Handling it safely means treating it as you would any lab chemical: thoughtfully, cleanly, and with just enough healthy suspicion to never forget the basics.
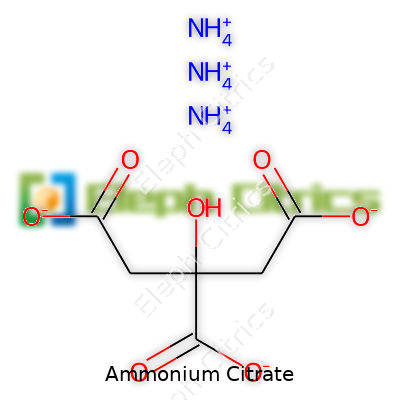
I still remember the first time I handled ammonium citrate in a lab. The label caught my eye: (NH4)3C6H5O7. Out loud, that’s three ammonium ions bonded with one citrate ion. Doesn’t roll off the tongue, but that formula tells you a lot about how these elements work together. It’s something many overlook, yet the presence of nitrogen and citrate brings together a mix of nutrition and function that gets used in everything from fertilizers to food processing.
Each part of the formula has a job to do. Ammonium delivers nitrogen – a staple for plant and microbial life. Citrate comes from citric acid, found in lemons and limes. So, ammonium citrate basically puts plant food and a natural acid together. That’s why people in agriculture prize it. It feeds crops with a slow, steady dose of nitrogen, reducing sudden changes in soil chemistry that can shock roots. The same formula sits behind products in countless labs and food facilities. Blender manufacturers use this compound to stabilize certain drink mixes, while photographers know it as a fixer in some black-and-white printing processes. Schools teach its formula because it highlights how organic acids bond with inorganic ions, helping students connect chemistry to something real.
Researchers continue to find new uses for ammonium citrate. It works as a buffer in biology, keeping solutions from drifting too far acidic or basic. This stability protects enzymes and bacteria that scientists want to keep alive. In the medical field, lab techs sometimes use it to help preserve blood or tissue samples, because that same stabilizing power prolongs shelf life. Farmers add it to fertilizers, letting crops use nutrients over weeks instead of days. Environmental experts appreciate its solubility; ammonium citrate dissolves easily in water, which means it’s less likely to stick to soil particles and more available to plants or lab experiments.
Just because a substance works well doesn’t mean there aren’t downsides. Ammonium compounds can sometimes lead to nitrate runoff. This contributes to water pollution if farmers apply too much or if rain carries it to streams. In my experience, best results come from targeted use: soil testing, careful measuring, and slow-release blends help limit waste. On the food side, some consumers worry about “chemical-sounding” ingredients even when they’re proven safe. Public education goes a long way. Chemists and nutritionists need to speak plainly about what each ingredient does. Open conversations about source and purpose keep skepticism at bay.
Understanding the formula of ammonium citrate means understanding why it ends up in so many products and processes. It’s not about memorizing letters and numbers. Seeing the function behind each element points to a world that depends on chemistry for food security, health, and innovation. Real progress happens when users – from farmers to students to cooks – see what’s in a bag, a bottle, or a beaker, then make smarter choices because of it.
Ammonium citrate sounds like something you’d run into only if you worked in a lab, but it pops up in food processing, fertilizers, and even cleaning supplies. When you spend your days around sacks of white powders, rules aren’t just written for the sake of having them—they save eyes, lungs, and sometimes entire businesses.
Let’s get to the root of real problems: moisture and contamination. Ammonium citrate pulls water from the air like a sponge. If you leave a bag open or cap a jar loosely, clumps start forming before you know it, and soon the whole batch might be unusable. Wet ammonium citrate can promote mold or react with metal, releasing ammonia gas with that unmistakable sharp smell. Not healthy or pleasant.
From my own time in warehouses, I saw good intentions unravel by one careless moment—a spill, a leaky roof, or someone using an old, cracked pallet. Loading chemicals on bare concrete or next to strong-smelling pesticides usually led to complaints from the quality inspectors. They were right: stray odors and moisture don’t just ruin samples, they can create unpredictable reactions.
Long before anyone brings in fancy equipment, the basics matter most. Tight-fitting lids and sealed bags—preferably made of plastic, not paper—stop moisture and cross-contamination. If you see condensation inside a container, that batch probably qualified for disposal in most professional kitchens or manufacturing sites I’ve worked in.
Shelving shouldn’t stand right up against windows or exterior walls. I remember a winter when a poorly insulated warehouse wall created a cold spot and ruined several bags stacked against it. Temperatures swing more than you think next to thin metal. Ammonium citrate likes steady, cool conditions: 15–25°C keeps it stable, but away from sunlight or heat vents. Every storeroom manager I’ve met keeps chemicals off the floor—wooden or plastic pallets actually make all the difference.
Chemicals need space from incompatible materials. Storing ammonium citrate near oxidizers or acids can lead to dangerous releases—nobody wants a surprise chemical reaction. Labels have to stay clear with no smudging, and expiry dates matter. Some people have a habit of ignoring those, but a simple walk-through and a checklist catch more issues than any expensive sensor.
Access should never be a free-for-all. Only trained staff should pull ammonium citrate from shelves, especially in larger quantities. I’ve seen the difference training makes: fewer spills, fewer accidents, and a safer place to work.
What would make things easier? Color-coded storage bins help in a fast-paced environment. Investing in humidity sensors and dehumidifiers may seem like overkill, but the cost of damaged materials racks up fast. Periodic checks with a moisture meter catch issues before they spiral, and regular refresher safety trainings keep old hands and new hires on the same page.
At the end of the day, storing ammonium citrate safely keeps people healthy and businesses out of trouble. Strong habits in labeling, sealing, and regular checks go further than any new gadget. There’s no shortcut for consistency, and that starts with everyone knowing why these steps matter. Facts aren’t just rules—ask anyone who’s had to throw away a day’s work because they didn’t pay enough attention.
Walking into any lab or industry where chemicals play a role, you hear about ammonium citrate. Chemists and teachers often talk about its two main types: monobasic and tribasic. Both carry the ammonium citrate label, but knowing their differences makes a real impact, especially for folks who use them in agriculture, food, or lab work.
Monobasic ammonium citrate comes with a single ammonium ion locked into the citrate structure. Tribasic brings three ammonium ions to the party. It’s not just a numbers game—this difference determines their acidity and how they interact with other substances. Monobasic carries a more acidic bite. Tribasic sits closer to neutral on the pH scale.
Curious how this plays out? Think about photo labs from old-school film days. Tribasic ammonium citrate ends up in developer baths, helping control the process without making the bath too acidic. On the flip side, monobasic ammonium citrate finds its place in tasks that benefit from a gentle acid touch—say, certain cleaning products or as a buffer in test kits.
Shifting to agriculture, soil scientists reach for each type based on what the crops need. Crops that don’t mind—or even like—a lower pH get monobasic. Tribasic takes its place where reducing harshness matters. I remember helping a friend with tomato soils. The test kit called for an acid buffer. The box listed monobasic. Sure enough, swapping it for tribasic changed the test results, throwing the numbers off for nutrient recommendations.
Any product that reaches food, pharmaceuticals, or fertilizers faces regulations. Each form of ammonium citrate brings different risks and safe-use levels. Monobasic can be more corrosive if handled the wrong way, irritating skin or eyes. People mixing large batches must pay attention to their protective gear. Tribasic, milder at the same concentration, sometimes takes preference in products where worker exposure is a concern.
Food manufacturers focus on purity and stability. Tribasic ammonium citrate gets the nod for certain applications because it resists breaking down and shifting the final product’s taste. Monobasic can play a role in food processing but often gets swapped for acidity regulation in things like drinks or jams, where a sour edge is desirable.
Synthetic production yields both types, but waste minimization matters, especially with growing environmental pressures. Some companies recycle by-products from other industries to produce either form, cutting down on chemical waste. Training workers in correct storage and handling of each type stays critical. A focus on clear labeling reduces mistakes—using tribasic instead of monobasic or vice versa can waste time, money, and even create unsafe conditions.
There’s room for better educational materials. People need accessible guides so labs, farmers, and students don’t confuse these chemicals. Digital platforms and QR-coded packaging open doors for easy-to-read instructions or MSDS sheets on the spot. Real-world examples and clear breakdowns of differences help prevent expensive or dangerous mix-ups.
Knowing which ammonium citrate to choose boils down to pH, intended use, and safety. These details shape fields from agriculture to industry. The best results come from a clear understanding of differences, up-to-date training, and practical, on-the-job experience.
| Names | |
| Preferred IUPAC name | Ammonium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Ammonium 2-hydroxypropane-1,2,3-tricarboxylate Triammonium citrate Diammonium citrate Monammonium citrate |
| Pronunciation | /əˈmoʊniəm ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 3458-72-8 |
| Beilstein Reference | 3599612 |
| ChEBI | CHEBI:63016 |
| ChEMBL | CHEMBL1201551 |
| ChemSpider | 5954 |
| DrugBank | DB11761 |
| ECHA InfoCard | 03e6be19-eae0-47b7-972c-fed1df5a4636 |
| EC Number | 208-627-6 |
| Gmelin Reference | 9187 |
| KEGG | C18457 |
| MeSH | D000662 |
| PubChem CID | 129703 |
| RTECS number | GE1750000 |
| UNII | DJ48GJ76N8 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID5040730 |
| Properties | |
| Chemical formula | (NH4)3C6H5O7 |
| Molar mass | 243.18 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.5 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.55 |
| Acidity (pKa) | 3.1 |
| Basicity (pKb) | 8.8 |
| Magnetic susceptibility (χ) | -48.0e-6 cm³/mol |
| Refractive index (nD) | 1.50 |
| Dipole moment | 0.0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.4 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | A12GA05 |
| Hazards | |
| Main hazards | May cause respiratory tract, eye, and skin irritation. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid breathing dust. Use only with adequate ventilation. Wash thoroughly after handling. Wear suitable protective clothing, gloves, and eye/face protection. |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 1, Instability: 0, Special: - |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 (oral, rat): 940 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 3000 mg/kg |
| NIOSH | TLV: 10 mg/m3 (as ammonium salts) (NIOSH REL) |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.2 – 2% |
| Related compounds | |
| Related compounds |
Citric acid Monosodium citrate Disodium citrate Trisodium citrate Ammonium sulfate Ammonium chloride |