Ammonium ferric citrate has followed a winding path through scientific and industrial history, surfacing first as chemists unraveled the secrets of iron salts. It found early fame in the photographic blueprint process—cyanotype—which dates back to the 1840s. Anna Atkins, a pioneering botanist, used it for some of the first-ever photographic books. As discoveries in medicine, food fortification, and environmental remediation rolled in, this compound evolved from a laboratory curiosity to a production workhorse. Along the way, generations of scientists tinkered with its crystalline forms, iron concentrations, and preparation methods, broadening its reach far beyond its Victorian roots.
Ammonium ferric citrate presents as a complex salt that’s more than just a mix of three ions. It offers a reliable, water-soluble iron source for labs, manufacturers, and researchers. Out in the market, it shows up as green or brown crystals and comes with distinct grades: pharmaceutical for supplements, technical for photography, and reagent for lab use. Whether it goes into a bottle of tonic, a developer’s mix, or an environmental test kit, its function changes with purity, iron content, and color.
At room temperature, ammonium ferric citrate typically appears as green or reddish-brown crystalline powder. Its solubility in water ensures swift integration into solutions at varying concentrations, supporting a host of applications. Chemically, it possesses a variable formula depending on preparation, but iron makes up about 16%–19% by weight in most commercial products, with citric acid binding the iron in a trivalent state. Unlike simple iron salts, the citrate groups reduce astringency and boost stability. Its stability in air outpaces certain other iron salts, avoiding rapid oxidation and caking that can disrupt lab procedures.
Proper labeling of ammonium ferric citrate remains vital, especially where health or research standards demand clear identification. The best manufacturers print batch numbers, iron content by weight, color grade, and grade specification (USP, BP, food grade) on each container. Technical data sheets usually detail trace metal content, pH range in water (typically between 6 and 8), and limits for heavy metal impurities. These details ensure researchers and technicians know exactly what lands in their beaker, supplement capsule, or developer bath.
To make ammonium ferric citrate, you start with ferric chloride and citric acid, dissolving both in water at an elevated temperature. Ammonia slips in to neutralize the solution, precipitating iron as a citrate complex instead of iron hydroxide. The resulting solution clears a deep green or brown hue, and slow evaporation allows crystals to form. Process control shapes color, crystal size, and iron content. Change the balance of ammonium to ferric ions, or raise the temperature, and production pivots from a green product favored in medicine to the brown variety used in blueprints.
In water, ammonium ferric citrate supplies iron(III) ions wrapped inside a stable citric acid framework. This whole structure shields the iron from immediate reduction or hydrolysis. What stands out is its photochemical action: under ultraviolet light, the iron(III) ion reduces to iron(II), giving up its place. This behavior underpins blueprinting and some water purification tricks. In medicine, this complex mitigates iron’s harshness in supplements by slowing transit and allowing gentler absorption. Modify the citric acid ligands or swap in a different anion and the complex’s solubility and reactivity change, letting chemists customize it for new uses in remediation or imaging.
Ammonium ferric citrate travels under several names: Ferric ammonium citrate, Iron(III) ammonium citrate, and ammonium iron(III) citrate. On the shelves, manufacturers sometimes market it simply as “F.A.C.” or “ammonium ferric citrate (green/brown).” These aliases crop up in patents, research papers, or ingredient lists, so anyone handling regulatory documents or supply orders ought to double-check which variety is on offer. Confusion among different iron-containing compounds sometimes leads to mishaps in food or pharmaceutical production—an error that tight labeling and clear communication help prevent.
Dealing with ammonium ferric citrate means understanding a few critical safety concepts. Though less risky than many iron salts, ingestion in concentrated form stresses the digestive system and may cause iron overload. Skin or eye exposure sometimes sparks irritation, so gloves and goggles make sense in production lines, labs, or blending. Storage containers should stay dry and sealed—moisture kicks off caking or slow breakdown, and the compound should be kept out of reach of children. Safety datasheets from major producers outline these steps and provide spill-handling guidance. Many plants run annual safety drills to reinforce these standards and keep workers aware.
Food, medicine, photography, and water treatment facilities all use ammonium ferric citrate in unique ways. In pharmaceuticals, its mild taste and solubility ease iron deficiency treatment, causing less gastrointestinal upset than ferrous salts. Food fortification, especially in developing countries tackling anemia, draws on its ability to disperse into beverages and powders. Medical imaging and diagnostic laboratories depend on its reducing action in specialized assays. Blueprinting, fading in commercial importance, still sees hobbyist and art use thanks to the rich blue tones of cyanotype prints. In water purification, it acts as a chelating agent or oxidant, binding heavy metals for removal. Each domain plays to the compound’s balance of effectiveness and mildness.
Ongoing research seeks more than just new uses for ammonium ferric citrate—it also hones existing applications. In the food sector, scientists investigate forms that minimize metallic aftertaste, improve absorption, or reduce allergic potential. Water engineers tweak the formula for better heavy metal scavenging in polluted river systems. Medical researchers probe whether modified forms support gentler iron delivery in chronic illness—such as anemia in kidney disease. Industrial teams look at scaling up eco-friendly processes, cutting down waste and boosting yield, while universities test nanoparticle variants for antimicrobial surfaces or fuel cell catalysts.
Iron compounds always stir concern about toxicity—too much iron from supplements can tip over into poisoning, especially for children. Ammonium ferric citrate skirts some dangers by binding iron more tightly, making overdose less likely than with other iron salts. Still, high intake causes fatigue, organ transplant risk, and gastrointestinal damage. Toxicology research continues monitoring blood iron levels, liver markers, and long-term side effects among patients and food consumers. Animal studies often seek upper safe limits for chronic exposure. As a rule of thumb, clear dosing guidelines and smart storage habits trump after-the-fact treatments. Emergency rooms see fewer cases involving ferric ammonium citrate than ferrous sulfate, likely due to its patient-friendly profile and lower use outside medical supervision.
Prospects for ammonium ferric citrate tie in with trends toward sustainable chemistry and targeted therapeutics. Scientists run trials pairing it with nanotechnology, hoping it might shuttle drugs or imaging agents directly to cells. Food engineers push for iron-fortified nutrition with fewer side effects, addressing global malnutrition. Water managers develop new methods for passing polluted runoff through tailored iron-citrate beds, snaring hazardous metals before they hit crops or drinking supplies. In each field, transparent research, broad collaboration, and strict safety standards matter more as applications widen. This compound’s long history continues to spark new solutions, driven by genuine need across medicine, manufacturing, and environmental management.
Anyone who’s walked through a hospital hallway has passed shelves lined with bottles of iron supplements. Among these, ammonium ferric citrate helps treat iron-deficiency anemia. Doctors prescribe it when the body struggles to make enough healthy red blood cells. Unlike some iron compounds, this stuff dissolves well and doesn’t clog up the digestive system as much. Nurses and caregivers usually notice fewer complaints about upset stomachs, which helps patients stick with their treatment long enough to make a difference.
There’s also its role in imaging the gut. Before an X-ray, radiologists use certain iron compounds to outline parts of the intestines. Ammonium ferric citrate stands out because of the clean view it gives, and even pediatricians trust it for younger patients. Studies in “The Journal of Pediatrics” point out its safety compared to older choices, which makes a difference for parents watching over their kids.
Not every food colors itself. Without iron fortification, baked goods and cereal wouldn’t support folks who cut back on red meat or greens. Food manufacturers turn to ammonium ferric citrate as a food additive, especially to boost iron content. It slides into products because it barely has a taste and doesn’t change how food looks. The World Health Organization considers it safe in small quantities, though buyers should still keep an eye out for overuse just in case.
Engineers and architects used to rely on blueprints just as much as rulers. Ammonium ferric citrate made those deep blue designs possible. In the world of cyanotype printing, light reacts with this compound to create striking images. Even today, some artists and hobbyists reach for it when they want to handcraft something unique—like prints on paper or fabric. Watching the colors change is a small reminder that chemistry shapes art as much as science.
Plenty of folks never think about the chemicals behind clean tap water. Ammonium ferric citrate helps remove heavy metals such as arsenic from water supplies. Water treatment plants add it to bind unwanted particles so filters can pull them away before the water reaches home faucets. The Environmental Protection Agency points to this kind of chemistry as a simple way to improve public health, especially in places facing old pipes or unreliable sources.
Iron supplements should be safe, food should nourish, and water should be clean. With ammonium ferric citrate, a few risks sit in the background: high intake causes problems, and the compound’s waste can build up if factories aren’t careful. Regulators keep a close eye on this. Most facilities follow procedures to handle it safely, but pushback from local communities can spur companies to invest in better filtration or switch to greener ingredients.
Some researchers want to replace ammonium ferric citrate in certain processes. They’re testing plant-based alternatives in food fortification or exploring new non-toxic materials for art and water purification. It’s early days for these discoveries. Until those ideas mature, ammonium ferric citrate stays on the job—quietly fixing iron shortages, supporting clean water, and leaving blueprints for the future.
Ammonium ferric citrate pops up in more places than most folks realize. It shows up in medicine, sometimes as an iron supplement, and even works in some foods as a food additive (E381). People sometimes see the green or brown powder listed in ingredient lists, but the average person may not know what it actually means for their health. With so many chemical-sounding names swirling around food, no wonder folks pause at something like ammonium ferric citrate.
Doctors and nutrition scientists have spent years studying iron salts because iron deficiency stands as a real and global issue. Ammonium ferric citrate breaks down to provide iron that the body can use, and it’s easier on the stomach than some other iron supplements. Research from the World Health Organization and peer-reviewed nutrition journals points out that, when used in proper doses, ammonium ferric citrate has a good track record for safety. Nausea or tummy aches can happen if someone takes too much, but the same holds for most iron supplements.
Food authorities in Europe and the United States approve ammonium ferric citrate as a food additive. The Joint FAO/WHO Expert Committee on Food Additives listed it as safe as long as intake stays within recommended limits. Food safety groups keep an eye on additives like this, so alerts come quickly if studies ever reveal new risks.
Low iron zaps people’s energy, weakens the immune system, and drags down concentration. Pharmacies carry all sorts of iron pills, and folks with anemia rely on doctors to help them get enough iron without side effects. Most people rarely need to think about iron overload, but certain health conditions make doctors keep a close eye on anyone taking iron for the long haul.
Food fortification with iron, using compounds like ammonium ferric citrate, helps in parts of the world where meat isn’t always on the menu. Millions have avoided anemia and developmental delays from iron-enriched foods. Looking back at my own work with nutrition clinics, families ask about “weird” names on packages, especially for their kids. The answer always comes down to balancing benefits and risks.
No chemical in food works as a free-for-all. Dose matters. Doctors recommend iron supplements tailored to lab results, not guesswork. The European Food Safety Authority sets an acceptable daily intake for additives in the food supply, and companies have tight rules for how much they use. Side effects tend to show up at much higher levels than people would ever eat in everyday foods.
Whenever new research hints at problems, health regulators revisit their guidelines. In the 1990s, scientists flagged concerns about some iron salts causing gut irritation at high doses in supplements, prompting product changes. These actions show the checking and cross-checking that goes into keeping ingredients safe.
People worried about food additives can read product labels and ask questions at the doctor’s office. In my experience, most doctors feel confident recommending iron supplements, including ammonium ferric citrate, when the bloodwork calls for it. Folks with iron-overload conditions or chronic illnesses need a different game plan and close medical supervision. Pregnant women, teenagers, and people on vegetarian diets sometimes need extra iron, so guidance from a registered dietitian or doctor makes a difference.
Ammonium ferric citrate has the chemical formula (NH4)5Fe(C6H4O7)2. Sometimes you’ll see it written in slightly different forms, reflecting variations in composition because of hydration or differing ratios. In my college lab days, reading the label before using any chemical saved us from making mistakes, and with compounds like this, attention always revealed something new: different suppliers, different hydrates, sometimes even slight color changes.
The specific arrangement of atoms in ammonium ferric citrate sets the stage for how it works in real life. Take its role in medicine: doctors prescribe iron supplements for people with anemia, and this compound’s structure gives it high bioavailability and makes it easier for the body to absorb. Every component in the formula pulls its own weight. Ammonium ions help the body take up iron, while citric acid keeps it stable and helps with absorption, doing more than just bulking out a tablet.
Over the years, those of us who love photography came across this chemical in “blueprint” processes like cyanotype printing. High school chemistry teachers and artists both rely on that distinct formula to get consistent, deep Prussian blue prints every time.
Getting the formula right goes beyond passing a quiz in chemistry class. In food science, a lot rides on precision—the difference between a safe, effective iron supplement and an upset stomach could come down to the details of the formula used. Citric acid in the structure acts not just as a stabilizer, but it actually assists in taste masking when this iron salt finds its way into food fortification programs. Growing up in a household with kids, you only notice the value of iron in food when it’s missing. The wrong form, the wrong taste, a gritty texture—kids spit it out and the nutrition plan falls apart.
Water purification also depends on getting the chemistry right. Feld engineers standing beside muddy floodwaters employ ammonium ferric citrate for its ability to act as a flocculant, using the particular ionic arrangement in the formula to help bind impurities and make them easier to filter out. I’ve watched rural communities gain access to cleaner water simply because the right form of iron compound arrived in the supply crates.
Compounds containing iron can stain counters, clothes, and skin in a matter of seconds, as any lab veteran will attest. Handling ammonium ferric citrate in the lab or manufacturing plant requires respect for its reactivity because of the iron that sits in the center of the structure. There’s a good reason most bottles sport warnings—solutions corrode, powders dust up and irritate, and incorrect handling leads to safety incidents.
Addressing these problems comes from thorough training and simple tools. Color-coded containers, updated labeling, and clear communication turn dangerous situations into routine procedures. Sometimes what feels like overkill—daily spill drills or requiring gloves and goggles—prevents last year’s accidents from repeating. As research into green chemistry grows, I think we’ll see even safer ways to use complex molecules like ammonium ferric citrate, driven by a real understanding of the way each atom plays its part.
Few people think about storage practices for chemicals until poor storage causes headaches. Speaking from my hands-on days in a school lab, even familiar reagents like ammonium ferric citrate can cause issues if left unchecked. Despite its regular use in everything from food coloring to medical imaging, it reacts to less-than-ideal storage like a half-open bag of flour in a damp cupboard.
This greenish or brownish powder picks up moisture out of the air. Anyone who finds mysterious clumps in a container knows how much damage humidity causes. Ammonium ferric citrate needs a place away from steamy sinks, not on the nearest window ledge. Cabinets in air-conditioned rooms tend to work best, especially if kept well-sealed and out of the reach of sunlight. Manufacturers and chemical suppliers quickly agree: keep it in a tightly closed container and put it somewhere dry. That simple change saves money and maintains the powder’s quality over weeks or months.
This chemical doesn’t handle sunlight well. Excitement from photosensitive reactions belongs in the chemistry textbook, not on the supply shelf. Any direct exposure to sunshine or even strong indoor lighting nudges the compound toward unwanted changes, risking waste. I have seen once-usable bottles turn questionable because someone stored them on a bright shelf. Dark cabinets or opaque containers are the quiet heroes here. Labels face out, contents face in, nothing fancy—just practical old-school storage.
Heat sneaks up: a storage closet next to the boiler or directly above a radiator bakes away quality. At temperatures above room level, ammonium ferric citrate may break down slowly, compromising not only safety but also accuracy in its intended use. For labs and storerooms that run warm, consider relocating the chemical or using cooled storage on hot days. Regular monitoring with a thermometer can help catch overlooked heat sources.
Chemical safety isn’t just an item on a checklist. Over the years, careful storage habits have saved me from harsh chemical odors, ruined supplies, and emergency cleanups. The right labels—showing dates and hazards—reduce mistakes and keep products fresh. Workers or students shouldn’t have to guess what’s behind a bottle. I’ve seen accidents start with a simple mislabel, so clear, dated labels stay on every container, always.
Simple interventions keep ammonium ferric citrate in good condition. Use desiccants like silica gel to keep moisture low. Reinforce a culture where the last person to use the container reseals it, checks the label, and scans for leaks. Place containers on shelves designed to limit jostling, far away from food or drink.
Long-term, investing in dedicated storerooms equipped with climate controls and thorough record-keeping fights against chemical waste and contamination. Responsible disposal routines for expired or degraded material protect everyone’s health and keep the workplace legal and tidy.
Proper storage of ammonium ferric citrate doesn’t require new technology. It asks for consistent habits, decent containers, and vigilance. That simple blend gives schools, factories, and medical labs confidence in what they keep on their shelves, with fewer costly surprises.
Before taking any supplement or medicine, it's good sense to know what you’re putting in your body. Ammonium ferric citrate often turns up in iron supplements, lab work, and even food coloring. Some think of iron as essential for red blood cells, not as something that could cause trouble. Yet, things usually stay harmless until they aren’t. I’ve learned to check labels ever since a mild iron supplement gave me an upset stomach. That taught me every substance has a flip side, especially for folks with sensitive bodies or other medical conditions.
Iron can throw your gut out of balance. Upset stomach, constipation, and diarrhea crop up more often than companies admit. Nausea sometimes follows, especially if a person skips food before taking a dose. Dark stools can alarm people, but with iron supplements, that’s a pretty ordinary sight. You might also notice a metallic taste in your mouth. After several years dealing with different iron pills, I found sipping water helped with the aftertaste, but no trick makes it pleasant. Kids and older adults notice these problems a bit more than healthy adults, especially if the dose is too high for their system.
Anyone with allergies should pay extra attention. Severe allergic reactions may involve skin rashes, swelling, or breathing trouble. Though rare, I have a friend whose lips swelled up after trying a new iron supplement; she needed fast treatment. Ammonium ferric citrate can also irritate kidneys or liver in people who already struggle with those organs. With iron overload, which often slips past unnoticed, symptoms build slowly: joint pain, fatigue, chest discomfort, skin turning bronze. This risk rises for anyone with hemochromatosis or similar genetic issues. Lab tests sometimes cause confusion if someone doesn’t mention they’re using iron supplements, since it can skew bloodwork results.
Reading instructions lowers risk. Following the recommended dose matters, especially for kids or pregnant people. It’s smart to take iron with a bit of food if your stomach lurches at the thought. Keep supplements locked away from children. Iron overdoses can turn fatal, and it happens more often than most realize. Doctors suggest avoiding iron with antacids, dairy, or tea; these block iron from working well, making side effects pointless. Keeping an open line with your doctor challenges the temptation to “fix” low energy yourself.
Side effects show up more in people who double doses, have underlying conditions, or mix too many supplements at once. Healthcare should start with honest talks, not blind trust in advertisements. A simple blood test shows if you even need more iron. Switching brands, lowering dosage, or choosing a liquid form cuts down on gut problems for many. Research backs up the need for monitoring iron intake, since both too little and too much cause harm. If there’s a reaction, report it to your doctor or a pharmacist. Industry regulations exist for a reason—consumer feedback leads to safer practices and better products over time.
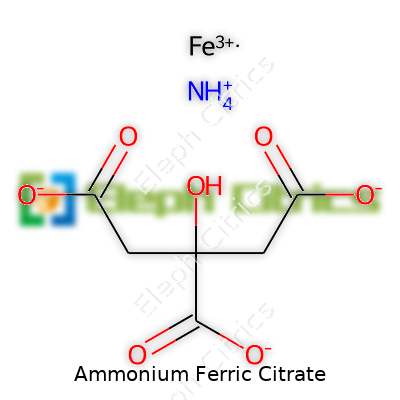
| Names | |
| Preferred IUPAC name | Ammonium 2-hydroxypropane-1,2,3-tricarboxylate iron(3+) |
| Other names |
Ferric ammonium citrate Ammonium iron(III) citrate Iron(III) ammonium citrate Citric acid, ammonium iron(3+) salt Ferric citrate ammonium |
| Pronunciation | /əˈmoʊniəm ˈfɛrɪk ˈsɪtrət/ |
| Identifiers | |
| CAS Number | “1185-57-5” |
| Beilstein Reference | 85339 |
| ChEBI | CHEBI:31638 |
| ChEMBL | CHEMBL1201640 |
| ChemSpider | 21538 |
| DrugBank | DB14487 |
| ECHA InfoCard | 03bafffa-4a76-4a6d-b258-2fd790df0ee7 |
| EC Number | 231-753-5 |
| Gmelin Reference | 15491 |
| KEGG | C18735 |
| MeSH | D000648 |
| PubChem CID | 159729 |
| RTECS number | SC5420000 |
| UNII | K19O93D77N |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID5047344 |
| Properties | |
| Chemical formula | C6H8FeNNaO7 |
| Molar mass | 449.17 g/mol |
| Appearance | Dark red powder |
| Odor | Odorless |
| Density | 1.81 g/cm³ |
| Solubility in water | Freely soluble in water |
| log P | -3.48 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.2 |
| Basicity (pKb) | 8.8 |
| Magnetic susceptibility (χ) | +2.4e-6 |
| Viscosity | Viscous liquid |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 274 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | B03AB05 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | Warning, H302, H315, H319, P264, P270, P280, P301+P312, P305+P351+P338 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| Lethal dose or concentration | LD50 oral rat 3000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): >2,000 mg/kg |
| NIOSH | NLK11207N3 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.2 mg/kg body weight |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Ferric citrate Ammonium citrate Iron(II) ammonium sulfate Sodium ferric gluconate Potassium ferric oxalate |