Benzyl lactate doesn’t usually appear at the top of anyone’s chemical wish list, but it’s been in labs for over a century in one form or another. Chemists first brought lactic acid derivatives into play because of their chiral nature and the way they help build more complex molecules. Benzyl lactate enters textbooks in the wake of esterification discoveries, where someone thought about combining benzyl alcohol and lactic acid to create something new for industry and research. The surge in organic synthesis during the postwar era really pushed interest. By the time green chemistry landed in the late 20th century, more eyes turned to lactic acid esters. Improvements in separation and purification ramped up the quality and opened the door to wider applications.
Benzyl lactate finds a home in the world of intermediates. Manufacturers turn to it for its mild aroma, good solvency, and compatibility with both water-based and organic formulas. The product usually arrives as a colorless to pale yellow liquid with a faint, sweet scent. Its role as a building block gets the most attention, sitting midway between raw chemicals and finished specialty compounds. You often find it next to bottles of benzyl alcohol or methyl lactate in supply rooms, but it stands out for its balance of solvent power and reactivity. Most buyers select it for prepping flavors, fragrances, and pharmaceuticals, given its food-safe origins.
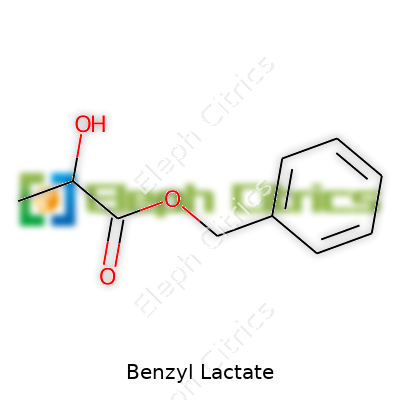
A look at benzyl lactate starts with the numbers: molecular formula C10H12O3, molecular weight 180.20 g/mol. The density floats near 1.15 g/cm3 at 20°C. You spot it boiling at close to 150°C under reduced pressure, which makes distillation straightforward but not without care. Its refractive index sits at about 1.5, a little higher than water but typical for moderate organics. The compound dissolves in ethanol, ether, and chloroform. It hydrolyzes slowly in water, thanks to its ester group. In practice, it feels stable on the shelf, but sunlight and acids can nudge it toward decomposition.
Labs need strict records. Benzyl lactate usually arrives labeled with its IUPAC name (2-hydroxypropanoic acid benzyl ester) along with a CAS number: 627-86-5. Manufacturers post purity on the bottle label, often above 98% for research applications, with specs for color, water content, and acid value. Labels warn against direct skin or eye contact and caution about flammability. Shelf life comes with instructions for cool, dark storage to keep it from breaking down. Regulatory info from REACH and OSHA typically triggers a safety data sheet at the receiving dock. Trace impurities, such as residual benzyl alcohol, get flagged. Everything gets tracked, especially in pharmaceutical or food projects.
Making benzyl lactate doesn’t call for exotic tools, just patience. Industrial players rely on esterification. They mix lactic acid with an excess of benzyl alcohol, using a catalyst like sulfuric acid. The recipe runs under reduced pressure and moderate heat, pulling water out to drive the equilibrium toward product. The process mimics classic Fischer esterification. Post-reaction workup involves neutralizing acid, separating layers, then distilling under vacuum to catch the pure ester. Chemists chase yields above 85% with enough purity to pass industrial standards. In green chemistry labs, folks experiment with solid acid catalysts and enzyme-driven systems to cut waste.
Benzyl lactate lends itself to transformation. That ester bond invites hydrolysis, snapping back to benzyl alcohol and lactic acid with the help of base or acid. Gentle hydrogenation can strike the benzyl ring, opening up routes to saturated analogs. Its primary alcohol group on the benzyl moiety reacts with halides and oxidants, making it possible to build more complex molecules. The lactic acid chiral center creates options in asymmetric synthesis, giving researchers a template for other chiral esters. In pharmaceutical chemistry, derivatization schemes often use benzyl lactate as a starting scaffold to hang more elaborate chains. You see it pop up in multi-step builds, especially when folks want to test out new protection and deprotection strategies.
People in the business know benzyl lactate by a handful of aliases. The IUPAC name spins out as 2-hydroxypropanoic acid benzyl ester, but shorter monikers stick better: benzyl 2-hydroxypropanoate, benzyl α-hydroxypropionate. Specialty suppliers add trade names, though most invoices call it by its main label. Safety data sheets highlight synonyms to avoid confusion during audits. None of these names change the underlying utility or legal requirements of the substance.
Every bottle of benzyl lactate comes with rules. Gloves matter—in the lab, the compound can irritate skin and eyes, and inhaling vapor leads to headaches and dizziness. Eye-wash stations and fume hoods turn accidents into minor stories. Spills stay manageable given its low volatility and easy cleanup with absorbent pads. Flammability stays low but not zero; storage away from open flames and oxidizing agents keeps mishaps at bay. Material safety data sheets tend to match those for solvents in the same family, focusing on quick response and proper PPE. Disposal goes through the standard hazardous organic liquid route, meeting EPA and local requirements.
Most benzyl lactate heads out to fine chemical plants, pharmaceutical research, and the fragrance world. Its mild scent and lactic backbone make it a handy solvent in some flavors and perfumes, where it brings out smoothness without overpowering other notes. Medicine manufacturers use it as an intermediate in building drugs that need chiral centers. The food industry taps small amounts for flavor formulations: its food-grade status means it slots in below toxicity alerts for trace additives. Cosmetic chemists use it to tweak emollient textures or as a solvent for active ingredients. Sometimes, it plays a role in lab-scale syntheses, giving researchers another lever for tuning reactivity and selectivity.
Work carries on to streamline production and widen the green credentials of benzyl lactate. Academic labs look for enzymatic paths that cut out mineral acids, aiming for a product with lower environmental impact and gentler process conditions. Drug developers spend time testing the ester’s utility as a prodrug component, since the lactic structure improves compatibility with biological systems. Material scientists keep testing benzyl lactate derivatives in polymers where biodegradability matters. Researchers studying asymmetric catalysis reach for benzyl lactate as a benchmark for selectivity studies in esterification and hydrolysis.
Most published work shows benzyl lactate as low in acute toxicity. Human exposure occurs mainly through inhalation or skin contact in industrial settings. Studies in rats show moderate irritant properties at high doses, but the compound clears from blood in a few hours, breaking down to lactic acid and benzyl alcohol. Chronic exposure hasn’t shown carcinogenic effects, but safety protocols aim to limit ongoing contact in the workplace. In cosmetics and flavors, regulatory bodies set strict upper concentration limits to stay far below adverse effect levels. Investigations continue into possible sensitization risks; so far, the data points to low reactivity. For waste disposal, no persistent byproducts turn up after proper incineration or biological treatment, which fits with its biodegradable profile.
The future for benzyl lactate looks tied to sustainability initiatives and new synthetic routes. More manufacturers turn toward enzyme-driven esterifications to drop process waste and energy use. As demand rises for biodegradable ingredients, both in packaging and personal care, benzyl lactate stands ready as a feedstock for specialty polymers. Ongoing projects explore its use in drug delivery, leveraging the way the body handles lactic acid and benzyl alcohol. In the tech world, niche applications in electronics and advanced coatings beckon, with tuning of the ester side chain opening up even more functional roles. As regulation tightens around solvent emissions and food safety, the focus sharpens on purity and traceability. Researchers and manufacturers keep their eyes open for ways to push benzyl lactate further—in both greener processes and endpoints that touch lives every day.
Benzyl lactate rarely gets a flashy headline, but anyone who's spent time reading ingredient labels in skincare or medicine might have stumbled on this quiet chemical. It looks simple—technically, it’s just an ester formed from benzyl alcohol and lactic acid. Chemistry aside, it’s found a practical home in personal care, pharmaceuticals, and even flavors.
You know the slightly tingly but soothing feel in a well-made lotion? Sometimes, that comes from ingredients like benzyl lactate. This compound delivers lactic acid without the harsh side-effects. Lactic acid belongs to the alpha-hydroxy acid (AHA) family, often seen in products that promise smoother, brighter skin. On its own, lactic acid can be a little too strong, so blending it into benzyl lactate softens the punch, maintaining results but lowering the odds of redness or burning.
Dermatologists understand how overuse of strong acids can lead people straight to dry, irritated skin. Finding balance is more science than luck—adding benzyl lactate achieves that middle ground. It lets the benefits of lactic acid happen gently, which makes it valuable to folks with sensitive skin who still want clearer pores or lighter dark spots.
Doctors sometimes ask for topical anesthetic creams that don’t sting, especially for children or people dealing with wounds. Benzyl lactate steps in as part of the solution, helping active drugs dissolve and spread evenly. Delivery matters: if a medication clumps up or dries before absorbing, it leaves people without the help they need.
In personal experience, even simple cuts or rashes are easier when a medicine soaks in fast and doesn’t sting. Medical research backs this up—emulsifiers and solvents like benzyl lactate improve both comfort and performance. The U.S. Pharmacopeia catalogues several creams that use it as an excipient, often paired with local anesthetics like lidocaine.
Many people never realize how much science goes into what we smell and taste. Food scientists reach for benzyl lactate because it carries a mild, sweet, floral note. A tiny bit folds into certain fruit flavors or perfumes, where it adds dimension without crowding out subtler scents.
The Food and Drug Administration considers benzyl lactate “generally recognized as safe” at the amounts found in foods. It helps round off rough edges in flavor formulas or lend complexity to perfumes. In my own kitchen experiments, a single unfamiliar note can transform a sauce from boring to memorable—this is the sort of chemical that often works those small miracles behind the scenes.
People have valid worries about unfamiliar chemicals. For benzyl lactate, safety reviews show it doesn’t trigger issues for most healthy adults in small quantities. Still, allergic reactions or irritation pop up occasionally, especially in those already sensitive to scents or preservatives. Reading the label matters.
Trust in ingredient safety depends on rigorous testing and honest reporting. Regulators like the FDA and scientific groups like the Cosmetic Ingredient Review dig into these substances, not just once but over decades. This is the backbone of consumer trust: knowing there’s a check on what goes into food and skincare.
Plenty of people want cleaner products, fewer synthetic chemicals, and clear labeling. Companies can focus on transparency, so shoppers know exactly how much of each ingredient ends up in the bottle or package. Scientists are working on alternates that share the strengths of benzyl lactate without the mild allergy risk. Honest dialogue—between consumers, companies, and regulators—pushes the entire system to do better.
Benzyl lactate pops up as a name on a handful of skincare and haircare ingredient lists. It’s not mainstream, but people curious about what goes on their skin often come across it. This compound acts as a skin conditioning ingredient and a fragrance fixer, combining the slightly floral scent of benzyl alcohol with the gentle acidity of lactic acid. Some manufacturers add it to lotions, conditioners, or topical creams, hoping to improve texture and create a smooth finish.
Researchers haven’t given benzyl lactate the same level of scrutiny as staples like glycerin or hyaluronic acid, but safety data does exist. Companies rely on the Opinion of the Scientific Committee on Cosmetology, plus toxicology studies on its two basic components. Benzyl alcohol appears frequently in leave-on and rinse-off products, while lactic acid is well known for its benefits and mild exfoliation. On their own, these chemicals prove safe in small doses.
A study published by the European Commission concludes that benzyl lactate, used at typical concentrations in cosmetic products, presents a low risk to human skin. The Cosmetic Ingredient Review Expert Panel in the U.S. also supports its safety when used as formulated in products, though they always stress patch testing new products at home, especially on sensitive skin.
Anyone who struggles with sensitive skin or allergies should stay cautious. Benzyl-based substances sometimes trigger irritation or mild redness. My own itchy rash after trying a new conditioner turned out to be an allergic reaction, so I’ve learned to scan for benzyl anything, not just benzyl lactate. This reaction isn’t common, but people with a history of eczema, contact dermatitis, or various fragrance sensitivities might want extra vigilance.
Reports of major reactions are rare. Dermatologists rarely see patients with benzyl lactate-specific issues, yet anyone with open cuts, or who uses strong medicated creams, should consult a pro before layering on new ingredients. Kids, pregnant people, and those with compromised skin barriers might want to skip products with new, less-tested compounds unless a dermatologist clears it.
Sustainability is now a regular part of product choices. Some companies derive benzyl lactate from natural sources (fermented sugar or plant oils), but others rely on synthetic routes. Unlike compounds like microplastics, benzyl lactate breaks down easily, so it doesn’t persist in waterways or hang around in the food chain.
Cruelty-free shoppers will want reassurance about animal testing. Major cosmetic firms in the EU and U.S. follow strict regulations that protect against unnecessary animal tests, but not all brands hold this promise worldwide. Transparency matters, so check the company’s stance before assuming.
The world of personal care isn’t getting any simpler. Each person’s mix of skin, preferences, and life experiences makes for unique needs. What works for your neighbor might not work for you, and “safe” on a label rarely means fail-proof. If you see benzyl lactate in an ingredient list, pause. A patch test on the forearm can help catch early warning signs of irritation before applying to the face or scalp.
Staying up to date on science, reading product reviews from real users, and leaning on the advice of trusted dermatologists always puts health first. If skin stings, itches, or breaks out, stop use and talk with a medical professional—no ingredient, no matter how promising, is worth discomfort or long-term trouble.
Benzyl lactate draws a bit of curiosity from folks looking into personal care products, flavors, or even pharmaceutical applications. It’s not one of those mystery ingredients with a confusing list of names. Its composition is straightforward. Benzyl lactate simply forms from two raw materials: benzyl alcohol and lactic acid. When the two combine, they create an odorless, slightly oily liquid that finds its way into a surprising number of products.
Benzyl alcohol comes directly from nature, but also from industrial synthesis. Roses, jasmine, and other plants produce it in small quantities. The cosmetic and pharmaceutical industries like it for its mild scent and gentle antiseptic properties. I’ve noticed it in topical creams and as a preservative in injectable drugs. What stands out is that it’s not particularly harsh on the skin, as long as you’re not allergic. Its structure features a benzene ring—a ring of six carbon atoms—attached to a single-carbon alcohol group. This combination gives it a pleasant aroma, making it popular in perfumes. For benzyl lactate, it’s more about being a functional building block than a fragrance.
Lactic acid, the other half of the duo, appears in many foods and skincare products. This compound, which many associate with yogurt and sour milk, actually gets produced by muscles during intense exercise. Industries manufacture it through bacterial fermentation of sugars. It lends tartness to foods, gently exfoliates dead skin cells, and acts as a pH regulator. Here, it meets benzyl alcohol, giving up a hydrogen atom and bonding together to form the ester known as benzyl lactate.
Mixing benzyl alcohol and lactic acid creates an “esterification” reaction. During this process, water gets stripped away. With heating and maybe a little acid catalyst, the alcohol group from benzyl alcohol hooks up with the acid side from lactic acid. Out comes benzyl lactate. I’ve worked in a small lab and seen how carefully you have to control this reaction. Even a slight shift in temperature can leave residue from one ingredient or the other, affecting product safety.
Consumers often skip ingredient lists, not realizing that knowing what goes into everyday products can aid in allergy management and support better choices for families. Lactic acid and benzyl alcohol both have lengthy track records, and reputable companies test for purity and safety. For people with sensitive skin, even natural components can trigger reactions, so it's smart to look behind the names on a package.
Chemists seek ways to reduce potential impurities during the production process. Better purification steps and cleaner raw materials help. The focus on “green chemistry” encourages minimizing solvent waste and using renewable feedstocks. That’s something regulatory agencies keep an eye on, especially in countries that demand strict traceability and safety for food and pharmaceutical ingredients.
Everyday products with benzyl lactate draw on just two basic ingredients, both well-studied and managed for safety. If you’re in the business of developing new personal care or food solutions, getting to know each raw material’s origin and potential contaminants can only help protect your customers. Transparency, careful sourcing, and rigorous testing are the cornerstones of a safer supply chain.
Benzyl lactate usually shows up in labs and industrial settings as a colorless or slightly yellow liquid. Folks use it as a solvent, a cosmetic ingredient, and sometimes even in flavors or pharmaceuticals. Benzyl lactate might sound obscure, though once you’ve handled enough chemicals, patterns start to jump out. Storage rules, for example, often rhyme between similar substances.
Chemicals like benzyl lactate aren’t just ingredients—they’re investments. Nobody enjoys junking a batch because it’s gone off from lousy storage. Keeping benzyl lactate in good shape starts with the right environment. Standard advice points to a cool, dry location. Temperature swings push liquid chemicals to break down or form unwanted stuff. Most manufacturers suggest aiming for room temperature, ideally below 25°C (77°F), but sticking closer to 15-20°C gives even better results if you can spare a fridge or climate control.
Moisture acts as a silent enemy. Once excess water sneaks in, benzyl lactate changes character and can lose value. Using tightly sealed bottles—preferably amber glass or high-quality plastic—stops water, air, and light from reaching the liquid. I’ve learned glass generally beats plastic for long-term shelving, though for fieldwork or shipping, plastic containers help prevent shattering accidents.
A well-labeled container saves endless headaches. Handwritten masking tape is a recipe for confusion during a late-night rush. Good labels tell you what’s inside, the concentration, and the date received or opened. This keeps the rotation system clear—nobody wants to use a bottle older than their last vacation. More than once, I’ve seen ruined experiments from folks grabbing unmarked or expired stuff. A simple inventory list, checked every couple of months, catches problems before they become disasters.
Storing benzyl lactate away from extreme heat and open flames counts for more than some might think. Spills near a heat source or sunlight risk dangerous fumes or spoilage. An overhead cabinet, away from windows or radiators, works best. Chemicals like acids and oxidizers react badly with benzyl lactate as well. Keep them separate, even if cupboard space feels tight. Back in my undergrad days, one misplaced bottle taught me that lesson fast.
Soaking up a benzyl lactate spill isn’t hard—an absorbent pad and proper disposal will do. Wearing gloves and eye protection proves worthwhile during cleanup or transfer. Over months or years, benzyl lactate can break down, so keeping it in tightly capped, full containers slows down this process, reducing contact with air inside the bottle.
No bottle of chemical lives in isolation. Keeping clear instructions posted—storage temps, emergency contacts, incompatibles—helps colleagues stay safe. Training new staff or students on these rules saves both dollars and danger. What works for one chemical works for many, but each has quirks that demand respect.
Getting storage right isn’t about following rules—it’s about building habits that protect product, people, and investments. By treating every bottle like it matters, risk shrinks. Proper labeling, sealed containers, and separation from hazards turn shelf space into trusted workspaces. That’s not just good practice; it’s common sense backed by real-life experience.
Benzyl lactate pops up in the ingredient lists for some creams, ointments, and even a few niche flavorings. Its job often lies in easing texture or carrying other ingredients. If you’ve never given it much thought, that’s normal. Most shoppers on a pharmacy run care about the brand or the main purpose of the product, not the compounds tucked away on the back label.
Still, even these less-noticed additives deserve a closer look. Growing up with eczema, I got used to checking ingredient lists because some seemingly harmless compounds would set my skin off. So I’ve learned to look for rare but real risks. Let’s dig into whether benzyl lactate causes trouble for folks using it on their skin or ingesting trace amounts.
Reports of benzyl lactate causing problems are rare in medical literature. For most people, using products with it goes smoothly, but a few issues have popped up. The most straightforward side effect is skin irritation, usually in people with sensitive skin or those dealing with open wounds. You might see redness, itching, or a slight burning feeling. These reactions line up with what you’d expect for any similar compound applied to fragile or broken skin. As someone sensitive myself, even a gentle ingredient can leave me scratching if I use it on raw patches. That makes label-reading worthwhile, even if the odds of irritation stay low for most users.
People with allergies to fragrances have another reason to stay vigilant. Benzyl lactate often shares chemical space with compounds that trigger allergic reactions, especially those known as esters. If you get hives or swelling from perfume ingredients, patch-test any cream with benzyl lactate first. So far, only a handful of cases suggest it causes major allergy problems, but they remind us to pay attention to even the so-called minor players in our personal care lineup.
If you use benzyl lactate as a flavoring, side effects get even less common. At the tiny concentrations used for taste, food safety authorities haven’t flagged it as a danger. But gut issues or mouth soreness can show up in people with extreme sensitivities, though such stories mostly crop up as rare anecdotes, not medical trends.
Most research on benzyl lactate circles around its safety for skin creams. The FDA and European food authorities rate it as “generally recognized as safe” for intended uses. Rigorous, long-term studies are a bit thin in the field. Short-term studies give us some confidence, yet they never rule out rare surprises. As someone who’s seen friends have odd reactions to “safe” products, I believe that practical research always trails behind real experience. One case report in a dermatology journal might not shake up the market, but if you’re the unlucky patient, that story matters.
Kids, the elderly, and people with chronic skin diseases probably feel the impact of any irritation more quickly. Pediatric and geriatric dermatology research lags behind that of average adults, leaving smaller safety nets for these groups.
If you know you have sensitive skin, patch-testing new products makes a lot of sense. Start with a dab on your arm and wait a day. Any itching, redness, or bumps gives you a clear signal to steer clear. Read labels closely and talk to a pharmacist or dermatologist if in doubt. I’ve learned, both personally and from working in a pharmacy, that asking never hurts and often saves you trouble.
Manufacturers could make things clearer. Detailed safety data, longer studies, and transparent labeling would help consumers avoid rare reactions. Until then, experience—your own, and what you can learn from healthcare professionals—remains your best guide.
| Names | |
| Preferred IUPAC name | 2-hydroxy-2-phenylacetic acid |
| Other names |
Benzyl 2-hydroxypropanoate Benzyl alpha-hydroxypropionate Benzyl lactic acid ester |
| Pronunciation | /ˈbɛn.zɪl ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 627-36-7 |
| 3D model (JSmol) | `/3D;coordinates;JSmol;C1=CC=C(C=C1)COC(=O)C2CO2` |
| Beilstein Reference | 1209282 |
| ChEBI | CHEBI:47494 |
| ChEMBL | CHEMBL3309073 |
| ChemSpider | 16212075 |
| DrugBank | DB14119 |
| ECHA InfoCard | 100.048.496 |
| EC Number | 211-290-7 |
| Gmelin Reference | 8246 |
| KEGG | C18695 |
| MeSH | D001581 |
| PubChem CID | 69601 |
| RTECS number | OD3897000 |
| UNII | I3H18L3NHI |
| UN number | UN1993 |
| Properties | |
| Chemical formula | C10H12O3 |
| Molar mass | 196.22 g/mol |
| Appearance | Colorless to yellowish oily liquid |
| Odor | Faint odor |
| Density | 1.16 g/cm3 |
| Solubility in water | Soluble |
| log P | -0.3 |
| Vapor pressure | 0.00022 mmHg at 25°C |
| Acidity (pKa) | 3.6 |
| Basicity (pKb) | 15.31 |
| Magnetic susceptibility (χ) | -7.68E-6 cm³/mol |
| Refractive index (nD) | 1.493 |
| Viscosity | 90.7 cP (20°C) |
| Dipole moment | 2.71 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 385.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -726.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3248.5 kJ/mol |
| Pharmacology | |
| ATC code | D02AE01 |
| Hazards | |
| Main hazards | Causes skin and serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 122 °C |
| Autoignition temperature | Autoignition temperature of Benzyl Lactate: 410°C |
| Lethal dose or concentration | LD50 (oral, rat): 2330 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Benzyl Lactate: "5000 mg/kg (rat, oral) |
| NIOSH | OD2800000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.2 – 1% |
| Related compounds | |
| Related compounds |
Benzyl alcohol Lactic acid Ethyl lactate Methyl lactate Benzyl acetate Benzyl propionate |