Butyl lactate isn’t a new invention. Its roots stretch back to the early drive for green alternatives, where farmers and chemists eyed surplus lactic acid from fermentation and started thinking about its chemical cousins. The world moved slowly from solvents made by distilling crude oil and started tinkering with ingredients from renewable resources. Industrial researchers found that butyl lactate, made from lactic acid and butanol, caught on with manufacturers looking to dodge harsh petrochemical cleaners in the mid-20th century. Over the decades, growing pressure for safer, greener, and less volatile compounds pushed demand for this and similar lactate esters. Laboratories and companies worldwide found new uses as environmental rules tightened and new regulations hovered on the horizon. Its journey from backroom chemistry curiosities to everyday cleaner ingredient says a lot about changing values in chemical production.
Butyl lactate brings a mild odor, slightly sweet, and an oily texture. It delivers strong performance as a solvent for paints, inks, coatings, and cleaners. Whether fresh from the lab or the warehouse drum, this clear liquid blends well with water and common organics. Unlike some older solvents, it pulls double duty, cleaning up residues without choking fumes or dangerous volatility. Chemical suppliers know it as a versatile option: compatible with both nitrocellulose lacquers and water-based systems. Companies plug it into degreasers, paint strippers, and specialty formulations that break up grime on machinery and tools. Product consistency keeps it sought after year after year.
On the physical side, butyl lactate stands out with a boiling point around 170°C, which keeps vapors down during normal use. Its specific gravity falls close to water but slightly heavier, giving it a robust feel when mixing. It won’t freeze under typical storage, with a melting point a little shy of -30°C, so drums stay liquid in winter warehouses. The molecular structure offers flexibility, pairing a lactate backbone with a butyl tail. This design lets it slip between dirt molecules and break them loose. Low vapor pressure holds risk to workplace air down, and the flashpoint sits safely above many standard solvents—a quiet nod to worker safety in busy shops.
Selling and shipping butyl lactate means following rules for labeling and documentation. Quality control checks water content, purity (typically above 98%), acidity, color, and odor. Each drum comes stamped with a batch number, manufacturer ID, and safety symbols required by global chemical standards. In the European market, compliance with REACH and CLP labeling laws is critical. North American drums include a GHS hazard pictogram, precautionary statements, and often a QR code linking straight to an updated safety data sheet. Every bottle and bulk shipment makes specs clear to avoid surprises at the mixing bench.
Butyl lactate emerges from a straightforward esterification reaction. Technicians combine lactic acid (often from cornstarch fermenters) and normal butanol in the presence of a catalyst. The setup heats the mixture until the two start swapping hydrogen and oxygen, forming butyl lactate plus water. Large-scale plants separate and recycle leftover water using vacuum distillation and add a drying step to polish the purity. Carefully controlled temperature, acid concentration, and residual moisture all sway the efficiency and final quality. Innovations in process intensification keep energy needs lower and waste down, a necessity for firms keeping a lean environmental footprint.
Chemists see butyl lactate as more than an end product. Its ester bond can snap open under alkaline or enzymatic action, breaking down into lactic acid and butanol once more—a useful trait for wastewater treatments keen to reduce persistent residues. For some niche markets, chemical modifiers adjust the chain, tweaking solubility or introducing new reactive groups. Reaction with oxidizers can slice it up, sometimes producing biodegradable byproducts that re-enter the natural cycle with less fuss than old-school solvents. This flexibility keeps research groups busy, looking for tweaks that push it into new market segments.
Butyl lactate wears plenty of different hats. Catalogs list it under butyl 2-hydroxypropanoate, lactic acid butyl ester, and 2-hydroxypropionic acid butyl ester. Brand names hop from country to country, reflecting language and distribution quirks. These alternate labels can trip up importers reading old documentation, so clear identification by CAS number (typically 138-22-7) saves time at the customs desk. In some markets, it appears in blended products under trade secret formulations—still, the core chemical slips in as everything from cleaning agent to low-odor plasticizer.
Chemical plants and jobsite managers keep a watchful eye on safe handling. Though butyl lactate ranks as less hazardous than many big-name industrial solvents, splash protection, good ventilation, and fire safety protocols matter. Safety data sheets highlight eye irritation and skin contact risks—gloves and goggles are non-negotiable in most shops. Storage uses sealed containers to prevent moisture pickup, and spill kits handle leaks quickly. Emergency response teams practice containment and neutralization using absorbents and cleaners built for organic compounds. Companies keep training regular, even as incident reports remain relatively rare. In the push for sustainability, clean disposal and recovery figure into site audits, checking that spent material heads to approved waste processors.
Factories and workshops turn to butyl lactate for cleaning, degreasing, and stripping jobs where performance and worker health collide. Automotive shops use it in sensitive paint cleaning and prep, where even a stray solvent whiff can put a technician in a bad spot. Printers, ink manufacturers, and packaging plants blend it into formulations for better flow, reduced smearing, and easier cleanup. Some personal care labs test it as a mild active for lotions, though purity requirements jump here. Food processors stay cautious; butyl lactate remains out of most food-contact products despite low toxicity, given regulatory caution. Environmental engineers see hope for site remediation and oil cleanup, thanks to its breakdown in nature without dangerous residues.
Across universities and company labs, butyl lactate pops up in green chemistry journals and patents. Scientists test it as a bio-based alternative in adhesives, epoxy systems, and specialty polymer synthesis—achieving high yield and fewer VOCs compared to standard petrochemical options. Research pushes for higher purity, process energy savings, and circular chemistry where byproducts feed into new cycles. Analytical teams explore rapid detection in waste streams to monitor for persistent organics. The search for improved catalysts and enzyme-based synthesis routes points to even greener production in coming years.
Over the years, health agencies ran butyl lactate through animal, cell-based, and human toxicity screens. Results routinely show low acute toxicity; most adverse effects link to undiluted contact or uncontrolled inhalation over long work shifts. Breakdowns in the environment trend toward complete mineralization, not bioaccumulation. Regulators keep reviewing chronic exposure reports, guarding against unseen effects, but most studies settle on classifications many steps safer than classic hydrocarbon solvents. As green chemistries grow, keeping a sharp eye on long-term, multigenerational studies remains a must, especially as usage volumes climb.
Industry observers expect butyl lactate to edge into more corners of manufacturing as strict regulations and market preferences keep steering away from hazardous solvents. Advances in fermentation promise cheaper, more sustainable lactic acid, making the economics better for bulk buyers. Expect tweaks to improve biodegradability, cut energy use, and make derivatives that fit specific high-value niche markets. As more research untangles the messy world of supply chains and low-carbon processes, butyl lactate sits in a strong spot for labs and manufacturers that want real progress in safety and sustainability, not just box-ticking.
Butyl lactate comes from lactic acid and butanol. It appears as a colorless liquid with a mild odor, often featuring in discussions around green chemistry. The solvent earned attention thanks to its low toxicity and friendly profile for workers and the environment. My own brush with butyl lactate happened in a manufacturing plant, where it stood out as a safer substitute for harsher chemicals.
Someone cleaning spray-paint guns or printing presses may not think much of the solvent swirling in their solution tank. Yet butyl lactate is there doing heavy lifting. Paint shops and printers use it to dissolve residues, making equipment turnaround easier and faster. It works well in coatings, cleaning agents, inks, adhesives, and textiles. For the automotive industry, this solvent frees up paint residues without destroying the plastics or finishes underneath.
Beyond paints and coatings, butyl lactate keeps showing up in products labeled "eco-friendly." That's because it easily replaces traditional, petroleum-based solvents. These older chemicals, like toluene or xylene, raise health flags due to their fumes and environmental issues. Butyl lactate offers similar cleaning strength but breaks down faster outdoors—it's biodegradable and less harsh to breathe.
I remember a shift working alongside a team where butyl lactate replaced a strong-smelling cleaner that left us coughing. Switching to butyl lactate eased that problem. Its relatively low vapor pressure means less is floating in the air, so lungs aren’t under siege throughout the day. OSHA fact sheets and European studies back up this worker-friendly reputation, pointing to much lower risk of long-term health problems.
Butyl lactate’s safety isn’t a coincidence. It comes from renewable resources—fermented sugars and natural alcohols—setting it apart from many legacy solvents. This origin means people who work with it avoid much of the toxic buildup that oil-derived products cause. Water treatment and disposal teams still need to respect it, but public data shows it breaks down before it can do much harm to wildlife or drinking water.
Cost keeps some companies from going all-in. As demand for bio-based chemicals grows, prices remain higher than for the fossil fuels they replace. Small manufacturers weigh these costs against the savings from easier handling and lower health risks. In my experience, many believe price will drop as production scales up, especially as larger companies set greener standards for their suppliers.
Performance can also be a sticking point. Butyl lactate tackles most grease and pigment, but some stubborn industrial residues call for stronger muscle. Chemists often blend it with other solvents to cover every cleaning scenario, a sort of “best of both worlds” strategy.
Chemicals like butyl lactate have shown how shifting to safer ingredients pays off. Companies and regulators need to keep moving the needle by incentivizing green chemistry research. Industry can back this up by sharing evidence from their own successful swaps. Communities and individuals benefit by asking what’s really inside our cleaners and finishes. Even small steps—like using products with green alternatives at home—can spark bigger changes.
Butyl lactate pops up in all sorts of workplaces these days. It’s a solvent in paints and inks, sometimes used in cleaning and degreasing. On the surface, it holds promise as a “greener” option than some older, harsher chemicals. A biodegradable label can ease worries, but trust built over years in labs and shops tells me there’s more to the story.
Common sense shines when handling chemical solvents. I learned this working in a small print shop years back. The label says one thing, but reactions tell another. Butyl lactate has a mild, almost sweet scent—at first. Spend enough time near an open container and headaches creep up, nausea starts knocking, and eyes begin to water. That’s not just paranoia; it’s the solvent affecting the central nervous system and respiratory tract. The CDC and NIOSH both warn about these symptoms, with studies confirming irritation even at low exposure.
Spills happen, even for careful workers. One cold Tuesday, a glass bottle slipped off a cluttered shelf. I wiped it up fast but skipped gloves since it “wasn’t anything strong.” Within minutes, my hands started tingling and redness appeared. Turns out, butyl lactate absorbs through the skin quicker than expected, and can cause dermatitis. No amount of hand-washing brings comfort once skin gets stripped by a solvent.
A peer once thought she could skip goggles while pouring thick liquid into the machine. A splash landed right under her eye—she reached for water, but the burning lasted all day. That sort of eye contact can lead to long-term irritation if not washed out quickly. The MSDS for butyl lactate reads clear: use in well-ventilated areas, keep protective gear on, flush eyes or skin with water for at least 15 minutes if contact occurs.
Training makes all the difference. People come and go in warehouses and labs, often learning by watching others. I’ve seen places cut corners to save money, but simple steps reduce risk. Good ventilation isn’t about fancy fans on ceilings; it’s about actually moving fumes away from breathing zones. Masks fitted to the nose and gloves made from nitrile or another strong barrier material do more for peace of mind than any printed data sheet.
Labels help, but real safety comes from habits. I’ve made a checklist for my own use: gloves every single time, goggles before opening any container, ventilation checked before even thinking about pouring. Supervisors hold the key—when they set an example, the rest follow. Health and safety audits spot problems before they turn into accidents. OSHA requires reporting and monitoring, which creates pressure for improvement, but only if everyone gets on board.
Solvents change, industries evolve, and new risks rise. People feel a need for “safer” replacements all the time. Choosing something like butyl lactate because it seems less harsh on paper shouldn’t mean letting your guard down. Resources like the CDC, NIOSH, and OSHA offer free guides and fact sheets—nobody should feel alone or in the dark. Creating open conversations at work, sharing mistakes, and double-checking instructions build the kind of culture where accidents get rare, not routine.
Butyl lactate, found in many cleaning products and used for its dissolving strength, brings much to the table in daily life and industry. The colorless liquid gives off a mild, somewhat fruity scent. Its pleasant smell alone can be a relief for folks tired of strong solvents in the workplace. On the surface, butyl lactate looks straightforward, but the details matter not just in science labs, but in any shop where it comes off the shelf.
The boiling point of butyl lactate sits at about 168°C (334°F), well above water. That means at typical room temperature, it stays liquid and stable, which matters for storage and handling. As for freezing, it solidifies below -64°C, a rare event unless working in extreme environments. Keeping these temperature numbers handy helps avoid accidents, especially if someone works with heating equipment or has to store bulk chemicals through changing seasons.
Density tells you how heavy a substance feels for its size. This solvent comes in at around 0.99 grams per cubic centimeter at room temperature, landing just below water. From experience, it pours much like water—neither syrupy nor runny like alcohol. Its viscosity, which measures a liquid’s resistance to flow, supports this easy pouring. These features help workers measure and mix it without much fuss; spills are easier to handle, and adjustments to blends are straightforward.
Butyl lactate blends with many organic solvents and breaks down in water better than some other esters. Its partial mix with water means it can help combine watery and oily ingredients in cleaning solutions or dyes. For businesses focused on greener processes, that water compatibility makes rinsing and disposal simpler. Instead of tough separation tasks, cleaning equipment can become as easy as a rinse with hot water and gentle scrubbing.
Unlike fast-evaporating solvents, butyl lactate evaporates slowly. With its low vapor pressure (about 0.24 mmHg at 20°C), it lingers in the open air, so fumes remain manageable. That slow evaporation matters in closed spaces—it gives workers time to react if a spill happens. Air quality stays steadier and less irritating compared to harsh, fast-evaporators, but keeping a ventilated workspace still remains essential.
Its flash point lands around 68°C (154°F). Many common solvents catch fire at lower temperatures, so butyl lactate cuts down fire risks. Still, safety training sticks with rules for all flammable chemicals. Store it away from open flames and keep chemicals capped after use. Following these rules saves property and lives; fire risks shrink when precautions get taken seriously.
Installing smart labeling and up-to-date ventilation helps reduce mix-ups and breathing troubles. Training workers to check the product’s boiling and flash points during handling can prevent most accidents. For those seeking better efficiency or eco-friendlier products, choosing solvents with higher water solubility and lower vapor pressure, such as butyl lactate, marks a good step forward.
Getting familiar with the physical properties of a liquid goes well beyond numbers. Knowing how butyl lactate behaves in the real world helps make workplaces safer and efficiency smoother. In my own work, swapping out harsher solvents for butyl lactate kept headaches down and messes easier to clean up. As we look at safer chemical choices, every bit of practical knowledge counts.
Butyl lactate pops up in plenty of industrial settings. Paints, coatings, cleaners—this chemical plays a big role thanks to its solvent power and biodegradable nature. Yet this usefulness comes with real responsibility. Some folks might not realize how tricky storage can get until something goes wrong. A leaky drum, an unexpected reaction, or a fire in a cramped storeroom makes the importance of proper handling impossible to ignore.
Years of working around chemical warehouses have taught me that nothing replaces good labeling, secure caps, and the right containers. Metal drums do a decent job unless they're made from reactive alloys. If stored in plastics, HDPE always beats out low-grade bins. Even one short-term mishap—an unsecured lid, a temperature spike—can create a cleanup nightmare. That’s not just theory; it’s been the reality for more than one warehouse team I’ve known. The fumes from Butyl lactate sneak up on you. A forgotten vent or blocked airflow quickly turns an average afternoon into an emergency.
Butyl lactate has a flash point around 123°C. That’s plenty high for safe handling, yet the real-world risks pile up in small, hot spaces. Direct sunlight heats up containers, making pressure build inside. On the job, I have seen warning labels ignored for the sake of convenience, with barrels left near windows or heat sources. Risk builds steadily, even in places with routine safety checks.
Storing drums or bottles out of sunlight, away from steam lines, and in areas with steady, cool airflow makes a big difference. My own rule: if you feel slick sweat on your neck while walking through the chemical area, things are too hot. Air movement matters, not just for temperature, but also to clear out those faint, fruity vapors. It doesn’t take much exposure for eyes or skin to burn or lungs to ache.
Even cautious teams get hit with leaks or spills. I saw one warehouse get a nasty surprise when rainwater seeped under a loading dock, mixing with spilled butyl lactate. The slimy mess triggered a slip hazard—and left everyone scrambling to grab absorbent pads and protective gloves. Sand or commercial absorbents make cleanup faster and keep chemical reactions in check. Easy access to eyewash and emergency showers is not optional.
Open flames, sparking motors, and even static electricity find fuel in spilled solvent. Sprinkler systems and fire extinguishers rated for chemical fires must stand ready. Regular fire drills let nobody forget how fast a normal day can shift into full-blown chaos.
Don’t trust generic storage advice or hope for the best. Training new hires to spot drips, sniff out unexpected odors, and ask about uneven flooring keeps everyone safer. Local laws spell out minimums—bonded storage, chemical-proof floors, bunded areas—yet my experience says teams who go further avoid more mistakes.
Inventory checks make sure old containers don’t fail without warning. Keeping detailed logs guarantees that mystery leaks get traced back fast. Team meetings, even five-minute huddles at shift start, help everyone focus on the risks without getting complacent.
Butyl lactate promises eco-friendlier cleanup and efficient thinning for industry. Used wisely, it brings plenty of benefits to paints, cleaners, and factories aiming to lower emissions. Skipping steps on storage, rushing shortcuts, or ignoring warning signs sets up expensive accidents, regulatory headaches, and shattered workplace trust. Keeping it safe pays off every single day. I’ve seen the cost of getting this wrong, and it always runs higher than the price of a well-built storage room.
I’ve spent years digging through reports on green chemistry, and butyl lactate comes up fairly often as a greener alternative to traditional solvents. Most people searching for an eco-friendly product want something that dissolves fast in nature, doesn't poison wildlife, and doesn’t stick around in rivers or soil. Butyl lactate, an ester made from lactic acid and butanol, checks some of those boxes — but there’s more to the story.
A 2005 OECD study showed butyl lactate breaking down by over 60% in less than a month in standard biodegradability tests. Microbes eat up the molecule, turning it into water and CO2. That sounds great — a product that doesn’t linger in the environment like solvents such as toluene. Real-world numbers confirm these lab results. If you spill a bit into soil or water, bacteria get to work before the month ends.
Here’s where experience in environmental risk work comes in handy: products labeled “biodegradable” often still leave a footprint, just smaller than their petrochemical cousins. Butyl lactate starts life as lactic acid, which gets made by fermenting sugars—usually corn or beets. Agricultural production uses land, water, and fertilizers, so a cleaner supply chain depends on smarter agriculture and less fossil-fuel energy during fermentation and transport.
Toxicity is another question that comes up a lot. You won’t find butyl lactate in the same risk league as benzene, but high concentrations can harm fish and aquatic bugs if spilled directly into rivers or lakes. Regulations in Europe and the U.S. put butyl lactate on the safer end of the spectrum for handling, but big spills or waste dumping still cause localized damage — and that’s something manufacturers hardly talk about when trying to look green.
I’ve followed some switchovers in the printing and coatings industry — swapping out harmful glycol ethers for butyl lactate-based blends. Plants often report lower emissions because butyl lactate does not volatilize quickly. Workers say their eyes and noses stay happier. The downside comes during waste handling. Biodegradability helps, but industrial users still need to keep disposal well-managed since too much of anything, even the greener stuff, can overwhelm wastewater treatment plants.
Locally produced feedstocks and renewable energy improve the cradle-to-grave story for butyl lactate. Choosing corn from low-fertilizer, no-till farms, for example, shrinks overall emissions. Manufacturers using closed-loop recycling for process water cut down on the mess left behind. Pairing these efforts with regular checks for emissions at plants keeps things transparent for neighbors and regulators down the street.
For small businesses or hobbyists thinking about switching to butyl lactate, the advice from years of environmental auditing holds: keep quantities in check, learn the risks, and don’t treat “biodegradable” as a license to pour waste down the drain. Instead, look for community hazardous waste programs or designated chemical pickups.
Butyl lactate signals a shift towards safer solvents in the market. Compared with traditional petrochemical cleaners and strippers, it cuts the risk for users and the community. Still, calling it fully “environmentally friendly” misses the bigger picture. Responsible sourcing, strict waste management, and transparency along the supply chain create the kind of product people trust in the long run. The details behind that “biodegradable” label matter just as much as the word itself, and they’re shaping how both industry and consumers think about what it means to go green.
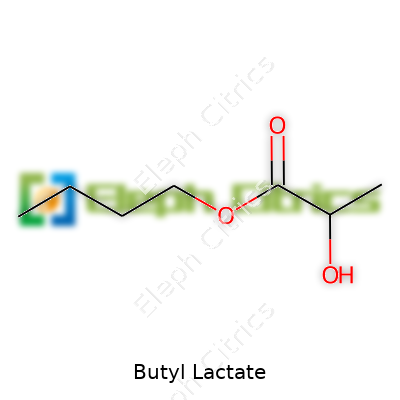
| Names | |
| Preferred IUPAC name | Butyl 2-hydroxypropanoate |
| Other names |
Butyl 2-hydroxypropanoate Butyl alpha-hydroxypropionate Butyl ester of lactic acid Butyl 2-hydroxypropionate |
| Pronunciation | /ˈbjuː.tɪl ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 138-22-7 |
| Beilstein Reference | **Beilstein Reference: 1721650** |
| ChEBI | CHEBI:31222 |
| ChEMBL | CHEMBL21737 |
| ChemSpider | 13125 |
| DrugBank | DB04022 |
| ECHA InfoCard | 13bab663-97a1-4ac8-abde-fb9c9b0cde2f |
| EC Number | 203-306-4 |
| Gmelin Reference | Gm. 3147 |
| KEGG | C18602 |
| MeSH | D017350 |
| PubChem CID | 8015 |
| RTECS number | OJ8580000 |
| UNII | 30YO44R1PU |
| UN number | UN2529 |
| Properties | |
| Chemical formula | C7H14O3 |
| Molar mass | **160.21 g/mol** |
| Appearance | Clear colorless to yellowish liquid |
| Odor | Mild fruity |
| Density | DENSITY: 0.92 g/cm³ |
| Solubility in water | partly soluble |
| log P | 0.84 |
| Vapor pressure | 0.19 mmHg (20°C) |
| Acidity (pKa) | 15.3 |
| Basicity (pKb) | 13.20 |
| Magnetic susceptibility (χ) | -8.44 × 10⁻⁶ |
| Refractive index (nD) | 1.407 |
| Viscosity | 2.8 mPa·s (20 °C) |
| Dipole moment | 3.24 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 373.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -600.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3030.7 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H226, H315, H319 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 124°C (255°F) |
| Autoignition temperature | 225 °C |
| Explosive limits | Explosive limits: 1.5% - 8.0% |
| Lethal dose or concentration | LD50 (oral, rat): 3730 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 3200 mg/kg |
| NIOSH | BUC |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Butyl Lactate: "5 ppm (skin), 25 mg/m³ (skin) |
| REL (Recommended) | 50 mg/m³ |
| IDLH (Immediate danger) | IDLH: 300 ppm |
| Related compounds | |
| Related compounds |
Lactic acid Ethyl lactate Methyl lactate Propyl lactate |