Calcium carbonate steps out of the pages of history as both a natural and industrial mainstay. Large blocks of limestone built ancient temples and pyramids; ready access to this rock shaped entire civilizations. People heated limestone to make lime for mortar, tamed acidity in soil with ground chalk, and even sculpted statues and artwork with marble. In the 19th century, the uses spread even more, with refinements in mining and purification techniques unlocking a fresh wave of industrial applications. Synthetic routes in laboratories followed, especially once the demand outpaced easy quarrying. To this day, the ancient touchstone of crushed rock continues echoing through everything from school classroom chalk to polished tablets in medicine cabinets.
You might hold a lump of chalk in your hand or stare out at a limestone quarry stretched across a hillside. That’s calcium carbonate—a product so familiar it hides in plain sight. Common names include calcite, aragonite, and vaterite, depending on the crystal structure. The ground-up powder ends up in toothpaste, paper, paints, supplements, medicines, plastics, building goods, even the food you eat. Manufacturers produce natural and synthetic grades, driven by demand for consistency, particle size, or purity. Tablets can be as refined as pure snow; crushed limestone may stick around in agriculture or concrete. Companies clearly label grades for industry, food, pharmaceuticals, or animal feed, with shared global standards helping keep quality consistent wherever it travels.
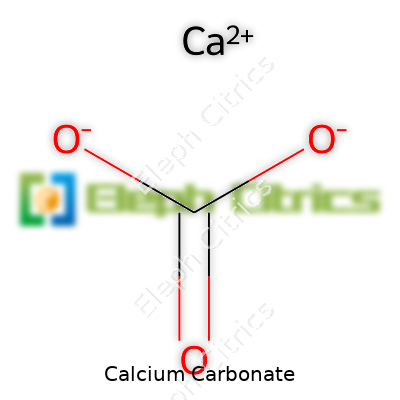
Calcium carbonate settles in as a white, fine to granular powder, though you find it as massive blocks of limestone or clear, rhombohedral crystals. The formula CaCO₃ marks it as a simple salt: one calcium, one carbon, three oxygens. It dissolves poorly in pure water but reacts quickly with acids, fizzing as it morphs into carbon dioxide. Hardness floats around 3 on the Mohs scale, so it’s soft enough for a pocketknife to leave a scratch. It weighs in at about 2.7 g/cm³ as a powder. Heat above 825°C and it breaks down, releasing carbon dioxide and leaving behind lime (CaO). Modern production demands tightly controlled particle sizes, shapes, and trace metal content for specialty applications like pharmaceutical coatings or cosmetics.
For every end use, detailed technical specifications walk step by step through the requirements. Industrial calcium carbonate suppliers must account for purity (usually over 98% for high-end applications), pH, loss on ignition, specific gravity, and residue left after burning. Particle size distribution can play a big part. For instance, a pigment manufacturer will want the average particle size and shape for proper paint coverage and brightness. In medicine, specifications get even more demanding—trace lead, arsenic, and other heavy metals fall below strict thresholds. Proper labeling supports transparency: batch codes, manufacturing dates, grade (food, pharma, industrial), and country of origin matter for recall tracking and regulatory compliance.
Natural calcium carbonate comes out of the ground. Miners blast limestone, crush it, and grind it into fine powders or granules as needed. Purity can pose a challenge with natural minerals, with impurities like clay or sand sometimes clouding the picture. Synthetic grades use the “precipitated calcium carbonate” process, reacting purified calcium oxide with carbon dioxide under controlled conditions to form a fine, consistent white powder. This approach lets manufacturers tune the size, shape, and surface area, making it more suitable for specialty uses in paper, plastics, and pharmaceuticals. Some plants run around the clock to keep up with growing demand from industries eager for fine, bright filler or raw material.
Calcium carbonate shows its versatility in the lab and factory. Exposed to strong acids, it foams and dissolves to release CO₂—an easy test to distinguish it from similar-looking powders. Heated in a kiln, it transforms to quicklime, used for everything from steelmaking to water treatment. Surface modifications further expand its uses, especially in polymer composites. Various surface treatments—like stearic acid or silane coupling agents—help calcium carbonate disperse within plastics and rubbers, improving mechanical properties or allowing better mixing. In the past two decades, new nano-sized variants have become available, offering increased reactivity and unique physical effects that interest material scientists and engineers.
Calcium carbonate wears many guises depending on context. Chalk traces school blackboards, marble graces kitchen countertops and art, limestone forms vast natural landscapes and crushed stone in concrete. Industrial buyers might read “ground calcium carbonate” or “GCC” on product lists; “precipitated calcium carbonate” or “PCC” signals a synthetic route. Other synonyms in the chemistry textbooks include calcite, aragonite, vaterite, whiting, marble dust, or oyster shell calcium. Due to wide interest and use, product names range from basic to highly branded, each focusing on grade, purity, or intended industry, and government regulations force clear distinction between food- or pharma-grade and industrial products.
Many people overlook the safety considerations with a substance as familiar as calcium carbonate. Dust, especially in bulk handling or manufacturing, can cause irritation to the eyes or respiratory tract. Especially fine grades need enclosed handling systems or well-maintained dust control equipment. Regulatory agencies like OSHA, the European Chemicals Agency, and food or drug regulators set maximum allowable impurity thresholds. The safety data sheets give clear instructions for storage, personal protection (like N95 masks for workers), spill response, and disposal. In medicine, absorption and bioavailability rates must meet health authority benchmarks, especially where the product lands in children’s supplements or antacid tablets. Continuous audits and standard operating procedures keep the product safe from quarry or reactor to shelf.
The reach of calcium carbonate covers an astonishing range. In construction, it forms the backbone of cement and mortar, while crushed limestone supports roads and rail beds. Papermakers use it to give a glossy finish and brightness to magazines, books, and cardboard. The paint industry calls for specialty grades that help pigment dispersal and whiteness. Plastics, rubber, and elastomers field specialty grades that lower costs and sometimes boost strength. Agriculture relies on it to amend soil, balance pH, and provide vital calcium. In foods, it acts as a calcium supplement and anti-caking agent. Toothpaste, antacids, and pharmaceutical tablets all take advantage of its mild, safe nature. Aquariums and water treatment plants lean on it to buffer water and maintain stable conditions. Cosmetic companies use superfine, high-purity powders for smoothness in creams and powders.
Researchers put a spotlight on calcium carbonate’s adaptability by looking for new applications and ways of processing or modifying its structure. Nanotechnology brings forward new forms, creating particles with controlled shapes and ultra-fine sizes. These materials find their way into medical imaging, pharmaceuticals, and specialty coatings. Scientists explore ways to improve solubility or create slow-release drug delivery systems using calcite as a scaffold. Environmental innovation drives research aimed at capturing and recycling carbon dioxide by converting it to synthetic limestone, which could lock away greenhouse gases for generations. Every year, new patents and studies signal enough creativity to keep this ancient mineral fresh and relevant for future industrial and health applications.
Most uses of calcium carbonate suggest a high safety margin, especially in consumer and food products. Regulatory agencies like the FDA in the United States or the EFSA in Europe carefully review bioavailability, absorption, and possible long-term risks. Excessive intake can cause issues—hypercalcemia can result from chronic overconsumption, leading to kidney stones or metabolic imbalances, especially in people with existing medical conditions. Occupational exposure focuses on fine dust inhalation, which may cause respiratory irritation with long-term or high-dose exposure. Stringent studies monitor trace contaminants like lead and arsenic. So far, leading research shows appropriate industrial grades remain essentially nontoxic for workers, consumers, and the environment, so long as regulations are followed.
The future holds fresh possibilities for calcium carbonate in both traditional and emerging applications. The drive for new building materials with a lower carbon footprint makes engineered limestone and upcycled demolition waste attractive feedstocks. Carbon capture projects harness industrial waste streams, aiming to convert CO₂ into valuable synthetic mineral products, locking carbon inside long-lived concrete or aggregates. Biotechnology start-ups experiment with bio-mineralization, emulating nature’s ways of making calcium carbonate for use in biomedicine, electronics, and synthetic bone. Researchers look for ways to tailor extremely fine forms to reinforce specialty polymers or composites, and to deliver minerals more efficiently in fortified foods and supplements. Sustainable quarrying, recycling, and high-purity production remain priorities as global demand keeps climbing.
Calcium carbonate isn’t the flashiest ingredient out there, but it reaches into almost every corner of daily life. At home, you’ll spot it on the back label of your chewable antacids. Anyone with heartburn has probably taken a couple of tablets containing this mineral. Doctors recommend it to help ease acid reflux. It also helps people keep their bones strong if they’re short on calcium. Dentists care about it, too. Toothpaste makers use calcium carbonate as a gentle abrasive, scrubbing away stains without damaging enamel.
Step outside and the uses keep going. Construction relies on calcium carbonate more than folks might realize. Walk past a new building and you’ll see cement bags and stacks of drywall. Both need calcium carbonate. Cement mixes start with limestone, which is mostly calcium carbonate crushed and baked at high temperatures. Drywall panels get their smooth white finish from gypsum, but many include this mineral as a strengthener. Whether a project is a backyard patio or a city skyscraper, this powder makes its presence known.
Newspapers used to leave hands covered in black smudges. Thanks to calcium carbonate, today’s paper feels smooth and crisp. Papermakers mix in this mineral to improve brightness and texture. The result? Clean, white pages that look sharp and hold ink well. Printing companies have come to rely on consistent batches of this material for reliable color quality.
In the world of paint, calcium carbonate works behind the scenes. Rather than paying for buckets of expensive pigment, manufacturers use it to cut costs without sacrificing thickness. That also prevents paints from sagging or running on walls. The pigment holds better, colors last longer in the sun, and surfaces keep their fresh look. Plastics makers follow a similar script, adding the mineral so products stay sturdy but light. Everyday items like shopping bags, bottles, and toys use it to keep prices down and improve performance.
Calcium carbonate matters well beyond city streets. On farms, it offers an answer to acidic soil. Crops struggle if ground is too sour, but spreading this mineral can bring pH levels back into balance. Yields go up, farmers make better use of fertilizers, and food ends up more nutritious. It even finds its way into animal feed, giving dairy cows and chickens the calcium they need for healthy bones, eggs, or milk.
Clean air and safe water depend on this unassuming mineral, too. Coal plants and factories use calcium carbonate to trap pollutants. Scrubbing acidic gases before they escape into the atmosphere cuts smog, acid rain, and health risks for people living nearby. Wastewater treatment plants add it to neutralize acidity and help metals settle, making it easier to filter out contaminants before water flows back into rivers.
Demand for calcium carbonate keeps rising. Some experts point out that mining and processing can create dust and emissions, so greener methods should get more attention. Recycled sources—like shells or slag from industry—offer a path with less environmental impact. At home, most people never pause to consider what’s hiding inside their antacids or toothpaste. Still, these everyday uses stack up. Good science and clear guidelines help keep products safe and effective, especially with dietary supplements and medicines. Choices about sourcing and safety will shape how this mineral helps both the planet and public health in the years ahead.
Growing up in a family where osteoporosis quietly crept up on older relatives, I learned early how important strong bones can be. Calcium shows up everywhere—your bones, your teeth, even muscles and nerves depend on it to work right. Yet, pulling that mineral from food alone proves tricky for lots of people. Enter calcium carbonate, that familiar white powder tucked inside countless supplement bottles.
Calcium carbonate comes cheap and packs a solid amount of elemental calcium into a small dose. Plenty of folks reach for it after a doctor draws blood and shakes their head at thinning bones. If your diet skips dairy because of an allergy, intolerance, or simply taste, supplementing seems straightforward.
It’s not just about bones, either. Athletes, pregnant women, and older folks rely on calcium because their needs shoot higher. Doctors hand out recommendations in milligrams—often around 1,000 to 1,200 daily for adults. Food often falls short. But calcium carbonate can plug the gap with a couple of tablets.
I once took over-the-counter calcium every morning, thinking more must be better. Soon enough, my stomach started throwing complaints. Turns out, calcium carbonate sometimes produces gas or constipation, since it needs acid in your gut to break down. People who already struggle with stomach problems might find it rougher than expected.
Swallowing too much means your body may get overloaded. Hypercalcemia—too much calcium in the blood—pushes kidneys to work overtime. Headaches, fatigue, and even irregular heartbeats sometimes follow. The National Institutes of Health pegs the tolerable upper intake at about 2,500 mg a day for most adults. Routine bloodwork at the doctor’s office can show levels headed in the wrong direction.
Taking calcium carbonate right after a meal helps your body soak it up—this tip came from my pharmacist, and it’s a small change that pays off. If you down it with a cup of coffee or right next to your morning iron pill, though, you get less benefit. Certain medications—like thyroid meds and some antibiotics—don’t mix well with calcium and lose their punch if taken together. Planning out your supplements with your health care provider makes a real difference.
Health experts agree: real food beats pills. Dairy, leafy greens, soybeans, and nuts offer more than just calcium—they deliver other crucial nutrients with every bite. Supplements act as backup, not a foundation, which is where I see many folks go wrong. Fortified plant milks, tofu, and even orange juice can bump up calcium without raising risk.
Daily calcium carbonate often benefits those with dietary gaps or medically confirmed low stores. Most healthy adults get by fine without a daily supplement, especially if they build meals with bone-friendly ingredients. For people at higher risk of osteoporosis or with proven deficiency, regular checks ensure doses match the need, and anything extra gets trimmed back.
Anyone worried about getting enough—or possibly too much—should have honest talks with their doctor, especially if health history already runs complicated. Balance stays key, both at the dinner table and in a supplement routine. Quality matters, so finding brands that earn third-party testing marks adds peace of mind.
People rely on calcium carbonate for a few big reasons: fighting off heartburn, treating acid indigestion, and giving their bones a boost by topping up calcium levels. It’s easy to pick up at any pharmacy, and doctors often suggest it for folks who aren’t getting enough calcium through diet. The flipside? Like any supplement or medication, it’s got a list of quirks that can catch users by surprise.
Ask anyone who’s used a calcium carbonate antacid about side effects, and tummy troubles usually come up first. Constipation tops the chart. Extra calcium in your gut slows things down. At times, people swing the other way and run into gas or bloating. I still remember an older relative who always kept chewable tablets handy, but then complained about getting “plugged up.” Turns out, she wasn’t alone—studies consistently point to slower digestion for regular users.
It sounds strange, but taking too much can actually cause pain rather than relief. High doses can raise blood calcium levels, especially in people with kidney issues. This can bring on confusion, muscle pain, feeling unusually thirsty, or even irregular heartbeats. The medical world calls it hypercalcemia. Hospitals see this most often in people mixing multiple supplements or mixing antacids with prescribed calcium pills.
Certain people, usually those with a family history, face increased odds for kidney stones when they regularly use calcium carbonate. The science blames excess calcium filtering through the kidneys and forming crystals—a rough situation I watched a friend go through. Doctors stepped in, slashed her supplements, and mapped out a diet with less animal protein and more water instead.
Not many folks realize how calcium carbonate interacts with other medications. It can lower the effect of some antibiotics or thyroid pills by binding with the medication before it gets absorbed. People taking medication for osteoporosis or high blood pressure also have to watch out for absorption issues. Pharmacists always warn me to put a couple of hours between taking calcium and swallowing other pills.
People with kidney disease need to step carefully—damaged kidneys can’t process extra calcium well, so smaller doses or different forms of calcium might be safer. For older adults who often juggle several medications, regular conversations with doctors or pharmacists go a long way. The same advice holds for anyone with chronic digestive problems like Crohn’s or celiac disease; what helps some can hurt others if doctors aren’t in the loop.
Most side effects can be avoided through a few practical steps. Spread calcium intake throughout the day to help absorption and keep things moving in the gut. Staying on the low end of the recommended dose sidesteps trouble for most people. Drinking plenty of water and staying active supports digestion and kidney health, two things that help keep calcium levels steady. Listening to your body and reporting changes early, especially muscle twitching, confusion, or stomach pain, gives doctors a chance to help before problems grow.
Calcium carbonate deserves its spot in many medicine cabinets, but it never hurts to double check how it fits your life and health. If you’re unsure, or you start noticing changes after adding a supplement, checking in with someone who knows the ropes makes a difference. The balance—getting enough calcium without overdoing it—depends on understanding how it acts in your system.
Plenty of folks want stronger bones or a way to fill the gaps in their diet. Calcium carbonate shows up almost everywhere—from kitchen cabinets to clinic shelves. I’ve seen many people just buy it over-the-counter because “calcium is good.” But products like these do more than sit there; your body has its own rules about how to use this stuff.
People often think that swallowing a pill means everything listed on the bottle just gets absorbed. The body doesn’t follow straight-line logic. Most of the time, calcium carbonate works best if you take it with food. Stomach acid helps break it down, making it easier for your gut to soak up. Swallowing a tablet after a meal or snack beats taking it on an empty stomach. I remember an older friend who used to pop his calcium supplement first thing every morning. Years down the line, his doctor noticed lower calcium levels, even though he took his pills religiously. He switched to taking them with breakfast, and the numbers in his bloodwork started to look better.
People see “more is better” stamped on a lot of nutrition advice, but calcium has its limits. The body does not handle giant doses all at once. Experts like the National Institutes of Health recommend not going over 500-600 mg per dose, since the gut handles small amounts much better. If a doctor says you should get more than that in a day, splitting the tablets boosts your odds of getting what you need. People who down high-dose supplements or eat lots of fortified foods run the risk of kidney stones, constipation, or even heart issues. The body stubbornly refuses to bend the rules, so keeping each serving sized just right keeps things safe and effective.
Doctors don’t just suggest calcium carbonate for bone health in older folks. People on certain medicines—like steroids or anti-epileptics—or those with digestive conditions sometimes struggle to get enough from food alone. I’ve seen some teenagers or pregnant women benefit from a supplement because their growing bones ask for extra. Still, it’s never a one-size-fits-all thing. Too much calcium from supplements, instead of food, links back to higher risks for heart disease in some research. So, asking a healthcare provider for advice fits better than guessing at your dose in the vitamin aisle.
Paying attention to what you eat helps. Vitamin D from sunlight or foods makes calcium work better, and lots of people forget that piece. Don’t take calcium carbonate with high-iron meals or certain thyroid medications—these can trip each other up inside the body. Drinking enough water with it also keeps those nagging side effects like bloating or constipation at bay. Listen to your gut, check in with your doctor, and keep things balanced. The simplest routines often bring the best results.
Many people grab calcium carbonate from the pharmacy shelf and toss it into their daily pill mix, rarely giving it another thought. It’s everywhere—antacids, bone health supplements, even multivitamins. But the truth is, calcium carbonate has the ability to change how other medicines work. I remember talking to a neighbor who combined her calcium chewables with her prescriptions. Just a simple mistake, but it left her wondering why her thyroid pills didn’t seem to have any punch.
Calcium carbonate does more than settle your stomach. It can block your body from soaking up certain drugs. Take thyroid medications like levothyroxine—calcium carbonate grabs onto these drugs and stops them working fully. This isn’t just theory; studies from major clinics have shown lower hormone levels in people who mix the two. The story repeats with antibiotics like tetracycline or ciprofloxacin. Calcium forms a tight bond with these antibiotics in your gut, making them less effective at fighting infections. Even heart patients taking digoxin have had hospital visits traced back to a simple calcium supplement dulling their medicines.
Swallowing calcium carbonate means more than adding a mineral to your body. It acts a bit like a magnet—latching onto certain drugs and dragging them out of reach. Researchers point to its ability to create insoluble compounds, turning once-absorbable pills into something your gut can’t use. It changes the stomach’s acidity, too, which alters how some medications dissolve or break down. This isn’t speculation—peer-reviewed medical journals document these effects, and pharmacists flag them for good reason.
Health pros don’t dig into your supplement list just because they love paperwork. With the average adult in the U.S. taking at least four prescription drugs, every addition brings a new risk of interaction. Surveys from hospitals show that nearly a third of patients forget to mention their non-prescription calcium tablets. Skipping this detail can cause everything from poor blood pressure control to persistent heartburn that never seems to improve. Doctors have seen folks struggle for months, only to find the source in their supplement cabinet.
Spacing out doses gives drugs a fighting chance. Taking calcium carbonate at least two hours before or after another medicine often keeps important drugs working properly. Pharmacies suggest timing schedules, and some even print reminders right on the bottle. People living with chronic diseases should keep a current list of all supplements and prescriptions. Apps now help keep track, and many clinics review these lists at every visit.
It pays to talk openly with doctors and pharmacists. Personal experience tells me that most will appreciate your attention to detail. The Food and Drug Administration, medical associations, and pharmacists’ organizations all recommend sharing every supplement you use, no matter how harmless it seems. The goal is better health, not more complications from what looked like a simple step toward stronger bones or calmer stomachs.
| Names | |
| Preferred IUPAC name | Calcium carbonate |
| Other names |
Alka-Mints Cal-Carb Calci-Mix Caltrate Oysco Tums |
| Pronunciation | /ˈkæl.si.əm ˈkɑː.bə.neɪt/ |
| Identifiers | |
| CAS Number | 471-34-1 |
| Beilstein Reference | 1363742 |
| ChEBI | CHEBI:3311 |
| ChEMBL | CHEMBL1377 |
| ChemSpider | 5290 |
| DrugBank | DB06724 |
| ECHA InfoCard | 05-211-683-263 |
| EC Number | 207-439-9 |
| Gmelin Reference | 85 |
| KEGG | D-calcium_carbonate |
| MeSH | D002121 |
| PubChem CID | 10112 |
| RTECS number | FF9335000 |
| UNII | 1VF76L70JP |
| UN number | UN2073 |
| Properties | |
| Chemical formula | CaCO3 |
| Molar mass | 100.09 g/mol |
| Appearance | white powder |
| Odor | Odorless |
| Density | 2.71 g/cm³ |
| Solubility in water | 0.0013 g/100 mL (25 °C) |
| log P | 0.00 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 9.0 |
| Basicity (pKb) | 8.3 |
| Magnetic susceptibility (χ) | -33.0e-6 |
| Refractive index (nD) | 1.658 |
| Dipole moment | 0 |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 92.9 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1206.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1207.6 kJ/mol |
| Pharmacology | |
| ATC code | A12AA04 |
| Hazards | |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Not classified as a hazardous substance or mixture. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 0-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 6450 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 6,450 mg/kg |
| NIOSH | CC1925000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 1,000 mg (elemental calcium)/day |
| Related compounds | |
| Related compounds |
Calcium oxide Calcium hydroxide Calcium bicarbonate Calcium chloride Magnesium carbonate Sodium carbonate |