Chemists started working with calcium citrate compounds in the late 18th and early 19th centuries, spurred by rapid progress in both chemistry and human health sciences. My own research into archival pharmaceutical texts reveals a clear interest in better ways to deliver supplemental calcium, especially after early discoveries highlighted the limitations of simple calcium salts. Over time, the shift towards crystalline hydrates, including calcium citrate tetrahydrate, reflected both technical advances and a growing appreciation for bioavailability. Today, this compound commonly appears in the pharmaceutical market, reflecting lessons learned from centuries of laboratory exploration and clinical trials.
This form of calcium citrate typically arrives as a fine, free-flowing powder. It solves quickly in warm water and has little taste, which probably explains its use in drink mixes and chewables in pharmacies and supermarkets alike. Many companies use it for more than just vitamin supplements; it's quietly tucked into antacids, food fortification blends, and nutritional meal replacements. Its widespread adoption shows how science can deliver practical answers for real nutritional problems.
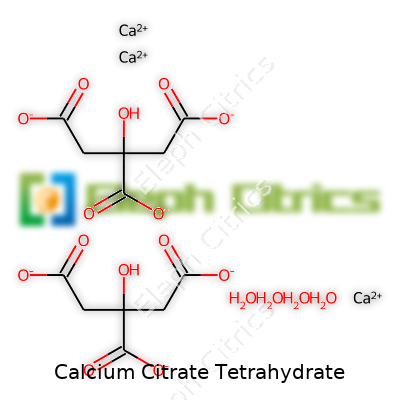
Looking closely, calcium citrate tetrahydrate presents as a white or almost white, odorless powder. Its chemical formula, Ca3(C6H5O7)2·4H2O, tells a clear story: each molecule binds water, offering stability and slightly increased solubility over its anhydrous sibling. It starts losing its water of crystallization as temperatures pass 100°C, a fact that’s not lost on process chemists working with heat-sensitive materials. The crystalline structure makes it easier to handle compared to some chalky or amorphous salts. Its pH in solution falls between 6 and 8, which helps both in palatability and compatibility in finished blends.
Most buyers look for specific benchmarks: assay results showing calcium content between 21% and 21.7% by weight, minimum purity over 98%, and strict limits for heavy metals and microbiological contaminants. Regulatory pressures call for clear labeling: each package needs a precise statement of calcium content, water of hydration, and any excipients. United States Pharmacopeia (USP) and Food Chemicals Codex (FCC) standards typically shape manufacturer protocols. Manufacturing facilities regularly undergo ISO or FDA audits, and companies can’t cut corners with documentation.
Manufacturers favor an aqueous reaction between citric acid and calcium carbonate. This approach releases carbon dioxide while calcium ions settle with citrate, forming the hydrated salt as the solution cools. Careful pH adjustment and controlled temperature prevent unwanted byproducts. Once the reaction reaches ideal parameters, filtration and washing remove remaining acids and minerals. Drying conditions, time, and humidity shape the hydration level, which affects both the handling and the shelf life of the final powder. This process comes from decades of fine-tuning, with operators drawing from both textbook chemistry and hands-on troubleshooting on the factory floor.
Calcium citrate tetrahydrate reacts as both a weak base and a weak acid, making it surprisingly flexible in more complex blends with other micronutrients or bioactive compounds. Acidic environments—like lemon juice or soft drinks—break it down into free calcium ions and citrate, helping its role as a calcium supplement. In some cases, food technologists use chemical modifications to adjust solubility or interaction with other ingredients, such as forming mixed salts with magnesium or zinc. The compound resists oxidation, which helps maintain product stability in the harsh environments of commercial food processing.
You may see calcium citrate tetrahydrate listed under several synonyms: tricalcium dicitrate tetrahydrate, E333 (in Europe), or simply calcium citrate hydrate on some food labels. Pharmaceutical catalogs often shorten it to “calcium citrate 4H2O,” a nod to its water content. Major ingredient suppliers rely on established chemical registries, which helps customers trace the compound’s source and verify its identity—an important step in today’s supply chain management.
Strict hygiene rules govern both the production and storage of calcium citrate tetrahydrate. Employees work with protective gear, and clean-in-place protocols are not optional. Spills or airborne dust don’t just threaten efficiency; unchecked dust poses a respiratory risk. Nevertheless, the compound scores low on acute toxicity, with an LD50 well above many household products. Regulatory agencies demand thorough traceability: product recalls triggered by quality lapses could cost millions, and no reputable producer will take those risks. Routine audits and staff safety training have become an industry norm.
Doctors, dietitians, and product developers rely on calcium citrate tetrahydrate because it outperforms other calcium salts in terms of absorption for certain populations. People with low stomach acid absorb it better than standard calcium carbonate, which changes the calculus for middle-aged adults. Food technologists use it to strengthen cereals, dairy alternatives, and juices, addressing calcium shortfalls in vegan diets or lactose intolerance. In the pharmaceutical world, it underpins chewable tablets and effervescent blends, chosen for its mild taste and chemical predictability. Even cosmetic scientists experiment with its mineralizing properties in toothpaste and topical skin-care blends.
Laboratories keep testing new delivery formats for this material, hoping to increase bioavailability or combine it with vitamin D for improved uptake. My experience collaborating with academic partners shows that constant improvement fuels product launches: controlled-release capsules, microencapsulated granules, and even functional beverages all draw on fresh laboratory insights. Analytical chemists keep refining assay methods, chasing down even trace levels of impurities, because subpar lots can undermine clinical trial results or regulatory submissions. Researchers also examine whether combining it with prebiotics might nudge absorption rates even higher, especially in the elderly.
Toxicologists have spent decades running animal studies and long-term monitoring in humans to understand the safety profile of calcium citrate tetrahydrate. Most studies support its safety when taken at recommended dietary levels. Excessive intake can raise risks of kidney stone formation, especially for people with previous history. Adding more magnesium often helps balance mineral intake and reduce those risks. Manufacturers monitor batch-to-batch variability in trace contaminants, as some naturally occurring impurities in source minerals could slip past if not properly controlled. Safety remains a keystone for continued research, and consumer trust relies on transparent study publication and open dialogue about both risks and limits.
Looking ahead, this compound will likely expand its footprint in both therapeutic and consumer markets. Personalized nutrition pushes companies to mix minerals in smarter proportions, and calcium citrate tetrahydrate forms a dependable base for custom formulations. Advances in plant-based foods and nutraceuticals open up new use cases; I see researchers getting creative with how it pairs with fibers or plant proteins to serve wider dietary needs. Regulatory frameworks may tighten further as digital traceability improves and consumer demand for clean-label ingredients intensifies. As global access to both supplements and fortified foods improves, calcium citrate tetrahydrate looks set to stay central in bridging nutritional gaps, especially in populations exposed to dietary fragility.
Growing up, I always heard neighbors talk about strong bones every time they mentioned a glass of milk. Calcium stands out in most kitchens, gym routines, and in the back of the minds of parents, especially when kids refuse to finish their plate of greens or dairy. The body uses calcium to build bones and support a healthy heartbeat. Without enough, bones lose their strength early on, and muscles work harder to keep routines running smoothly. It’s not only about what you eat; it’s whether your body can absorb the minerals in your food that makes the difference.
Calcium citrate tetrahydrate gives people an alternative, especially for those who don’t handle dairy well or find themselves with stomach issues from other calcium sources. This form dissolves more easily compared to other types like calcium carbonate. People often hear doctors mention it if acid reflux or digestive issues make swallowing pills unpleasant. Since this compound doesn’t rely on stomach acid for absorption, older adults, people using antacids, or anyone diagnosed with conditions that lower stomach acid use this supplement to latch onto calcium benefits.
For years, experts have pointed to the close link between calcium intake and reduced risk of osteoporosis. Women after menopause face an especially high risk of brittle bones. A study published by the National Institutes of Health found that regular use of well-absorbed calcium supplements, especially in postmenopausal groups, leads to better bone density. Calcium citrate tetrahydrate checks these boxes by not only boosting levels, but also by minimizing stomach upset.
Calcium citrate tetrahydrate has found its place beyond the supplement aisle. Food processing depends on precise formulations, and this form blends well into powders and liquids. Bakers add it to breads and baked goods, yogurt producers turn to it for fortifying their recipes, and cereal makers ensure every spoonful delivers real nutritional value. It slips into blended drinks, so people with milk allergies still get a calcium source in their daily diet. Its neutral taste means nobody notices it in their food, sidestepping that chalky flavor some other calcium powders bring.
Pharmaceutical teams have leaned toward this calcium salt not just for bone health pills, but in treating specific deficiencies. Hospital patients on restricted diets, pregnant women, or growing teens with high calcium needs benefit from products using calcium citrate tetrahydrate. This flexibility matters, especially as the world grows older and demands for preventative health measures keep rising.
A lot of people still struggle to meet the daily recommended intake of calcium, especially in areas where dairy products aren’t part of the culture or are too expensive. The numbers point out a continuing gap — recent surveys indicate up to half of teenagers and older women don’t meet their calcium targets, setting themselves up for long-term trouble.
Education helps, but solutions need to address availability and palatability. More food products fortified with well-absorbed forms like calcium citrate tetrahydrate bridge the gap for populations who can’t consume milk or cheese. Doctors play a key role in helping patients choose supplements wisely, steering them toward those that fit their health profiles. It only works if people stick with these choices, and that means finding convenient, affordable, and stomach-friendly options.
A lot of people might think of calcium citrate tetrahydrate as just another supplement or lab material, but it has real value in healthcare, food production, and even science classrooms. Like many mineral-based substances, the way you store it can make or break its effectiveness. If you keep it in the wrong spot, that powder can clump, degrade, or absorb stuff from the air nobody wants near their vitamins or food additives. So, knowing the right approach isn’t just some technicality – it protects quality, keeps people healthy, and saves money.
Think of storing calcium citrate tetrahydrate like protecting good coffee beans or flour. Moisture changes everything. That compound picks up water straight from the air. Humid storage turns a dry, free-flowing powder into a soggy brick. I’ve watched that happen firsthand in warehouse heat waves. The label said “keep tightly closed, store in a dry place,” but someone left the lid off. By the end of the week, the material was ruined, and that’s not just wasteful, it can throw off entire production runs.
Calcium citrate tetrahydrate thrives in a space that feels almost boring: cool, dry, shaded. Room temperature works for most non-industrial uses—think 15–25°C—but direct sunlight is a problem. That sun heats up plastic or glass containers, and before you know it, moisture condenses inside. Any excess heat can encourage that powder to degrade, especially if you’ve broken the manufacturer’s seal.
I’ve picked up pouches meant for chemicals that got soft over a few weeks. Turns out, thin plastic leaks moisture in slower than it leaks out. Airtight containers, preferably glass or thick, food-grade plastic, pay off in the long run. If you care about purity or using the product in food, check that the container actually seals tight. Silica gel packets can help; toss one inside to soak up stray moisture, and you’ll notice a difference in how the powder pours, even after months.
Most suppliers ship the powder in tough polyethylene bags or jars. Don’t just toss these on open shelves or leave them near cleaning supplies. Some industrial cleaners or even common kitchen spices let out strong vapors, which calcium citrate tetrahydrate can absorb. I once made the mistake of storing a sealed bag next to vinegar—no spill, just the fumes—and the powder smelled permanently off.
There’s more to safety than avoiding clumping. All food-grade calcium powders should be labeled with the production or expiration date. That information means something. Over time, the material slowly absorbs CO₂ from the air, or reacts if left open. I’ve seen expired calcium citrate turn hard, and no amount of shaking brings back the original texture.
So, toss any scoop you use for measuring into a clean, dry area, never leave utensils resting inside, and always re-seal between uses. Parents with kids at home should treat this compound with respect: store well out of reach, just as you would with any supplement or additive that could be mistaken for something else.
There’s nothing glamorous in tucking away a jar on a high, dry shelf, but mistakes here can cause serious headaches. A little attention to the basics—choosing a steady, moderate temperature, locking out the humid air, and using real sealing containers—keeps calcium citrate tetrahydrate effective and safe. In my experience, it’s the honest, sometimes boring storage habits that offer the surest guarantees in both home and lab settings.
Calcium supplements fill plenty of pharmacy shelves, but not all kinds of calcium show up as the same thing once in the gut. Calcium citrate tetrahydrate pops up in many daily multivitamins and dedicated calcium pills. It dissolves well in water and gets absorbed without a hitch, whether you’ve eaten or not. For people who struggle to digest other types of calcium, like calcium carbonate, citrate forms offer a real alternative.
Health professionals recognize calcium citrate as an effective source of dietary calcium. Medical research, such as reviews published by Mayo Clinic and Harvard Health, puts calcium citrate ahead of some other options for people with low stomach acid. Especially as folks age, the body’s production of gastric acid drops off, and that’s where calcium citrate makes a difference.
Most healthy adults land in the recommended daily intake range at about 1,000 to 1,200 milligrams of calcium per day from all food and supplements. Swallowing too much has never done bone health any favors, and scientists tie excess amounts to kidney stones or, in rare cases, calcium buildup in blood vessels. Big health agencies, including the National Institutes of Health, point out that both calcium from food and supplements count toward that daily maximum.
Calcium citrate comes in various hydration states, and the tetrahydrate form just holds more water molecules compared to anhydrous or monohydrate forms. This chemical detail bothers manufacturers more than it does typical shoppers, since it affects measured amounts. No extra safety risks pop up from those additional water molecules in tetrahydrate form. It’s the calcium itself, not the hydration, that plays the starring role.
Every supplement asks for some sense. Checking your own diet makes sense before adding any tablet. Most healthy folks eating dairy, fortified drinks, leafy greens, and fish with bones end up pretty close to enough calcium without much hassle. Some groups—postmenopausal women, older men, vegans, and people who can’t have dairy—fall short. Doctors often recommend supplements to round out those gaps, and citrate forms get the job done with fewer tummy troubles.
Reports on side effects usually focus on digestive complaints like gas or bloating, especially at doses above 500 milligrams at a time. Splitting up doses through the day helps avoid these problems. Patients with kidney trouble should talk clearly with their doctor first, since there’s a real risk for calcium to build up if the kidneys can’t keep up.
The real risk doesn’t come from calcium itself but from poor-quality supplements. Manufacturing slip-ups, impurities, and inaccurate dosing all turn a helpful supplement into a risky buy. Reputable brands, clear labeling, and third-party testing stick out as best practices. Some expert groups, like the U.S. Pharmacopeia, list trusted names who meet strict standards.
Anyone thinking about taking calcium citrate supplements day in, day out benefits from checking what’s already in their regular diet. Blood tests and a doctor’s advice always offer the most tailored guidance. Splitting doses, sticking to tested brands, drinking plenty of water, and keeping up with routine checkups all work together to make daily calcium citrate as safe as any supplement can be.
Calcium supplements fill store shelves with a line-up of names that sound like homework for a chemistry class: calcium carbonate, calcium citrate, calcium gluconate, and the less-familiar calcium citrate tetrahydrate. Most people walk away clutching the cheapest bottle or the one their neighbor swears by, without really having a grip on what sets these types apart. Yet, the details make a real difference for those hoping to support their bones, teeth, and nerves.
In my own family, my father needed extra calcium after being diagnosed with osteoporosis. We learned that not all forms break down in the gut the same way. Calcium carbonate demands a pretty acidic stomach to separate properly, which can cause trouble for older people or anyone taking medications like proton pump inhibitors. My dad switched to calcium citrate after struggling with stomach aches from calcium carbonate. He found the citrate form worked much kinder on his digestion—which lines up with research showing calcium citrate absorbs well, even when stomach acid runs low.
Calcium citrate tetrahydrate looks even more interesting in this landscape. This version adds water molecules to the crystalline structure, making it highly soluble. As a result, it blends more easily in liquids and becomes available to the body at a faster pace. For those with limits on stomach acid, or those who need reliable absorption—post-menopausal women, folks recovering from surgery, or older adults—calcium citrate tetrahydrate stands out for being gentle and effective.
Supplements that "go in one end and out the other" don’t help anybody. Studies back the idea that the body grabs hold of more calcium from citrate-based forms than carbonate. One study in the Journal of Clinical Pharmacology showed that calcium citrate blends, including tetrahydrate, helped participants reach higher blood calcium levels, meaning more of it got into the system where it can help bone health. Plus, the reduced risk for constipation matters to plenty of folks, especially elderly people managing several medications.
Calcium carbonate costs less at first glance, so large supplements in big-box stores usually choose it. But any savings evaporate if your system doesn’t absorb it, or if you stop taking it due to gas, bloating, and constipation. Calcium citrate tetrahydrate, being easier to absorb and lighter on the stomach, will cost a bit more, but the investment pays off in better results.
Choosing calcium isn’t only about cost or number of milligrams listed on the bottle. Consider your own digestive strength, your age, and your broader diet. For most adults, especially those facing any kind of stomach trouble or taking acid blockers, the advantages of calcium citrate tetrahydrate are hard to beat. Mixing it in smoothies or water makes it simple for people who can’t swallow pills. It fits well into busy lives, especially for those who don’t always eat dairy or leafy greens. Talk to your doctor or dietitian before changing supplements, but keep in mind: the best form of calcium is the one your body can truly use, not just the one that fills a bottle on a shelf.
Calcium Citrate Tetrahydrate pops up on the label of more than a few supplements these days. Doctors hand it out for folks with low calcium, and plenty of people reach for it hoping to shore up their bones or tackle muscle cramps. Compared with other calcium salts, it dissolves well, even for people with low stomach acid.
Stomach trouble ranks high on the list of gripes after starting calcium citrate tetrahydrate. I’ve watched friends complain of bloating, constipation, or mild abdominal pain. Taking more than the recommended daily dose only makes things worse. The National Institutes of Health point to constipation as a well-known reaction to calcium supplements. People might think constipation is nothing to worry about, but ongoing trouble can lead to real discomfort and even contribute to conditions like hemorrhoids.
Nausea shows up sometimes. It’s more likely if someone takes the pills without food or swallows a big dose all at once. Sometimes splitting the dose during the day helps keep that queasy feeling away.
Gas and a heavy feeling in the stomach usually settle after the body adapts, but not always. Every system reacts differently. Drinking more water and adding some fiber help some people handle these annoyances.
Too much calcium creates a new set of problems. Taking excessive supplements over months pushes up the risk for kidney stones. Doctors see this problem more often in folks who already get lots of calcium from their diet or have a family history of kidney stones. Keeping daily intake under 2,000-2,500 milligrams, as recommended by leading health authorities, can lower this risk. If you’re prone to kidney stones, a quick check-in with your physician makes a lot of sense before starting calcium supplements.
Excess calcium can also play tricks with the heart and kidneys. The Mayo Clinic talks about “hypercalcemia” — a mouthful of a word, but it means too much calcium in the blood. Symptoms include fatigue, confusion, excessive thirst, and oddly spaced-out feelings. I haven’t seen this come up among healthy adults who follow standard dosing recommendations, but there are reports in folks with pre-existing health conditions, especially those with kidney trouble.
Calcium supplements, especially in hefty doses, may mess with how the body absorbs antibiotics, thyroid medications, and some blood pressure pills. Doctors and pharmacists keep a careful eye out for this. Spacing out doses often sorts this problem. Tell your healthcare provider about all your meds before starting calcium citrate tetrahydrate, so you avoid those headaches down the line.
Choosing any supplement deserves solid advice from your healthcare provider, especially if you use other medicines or have health conditions like kidney issues. The food you eat does a lot of heavy lifting for your calcium needs — leafy greens, dairy, and certain fortified foods pull their own weight. If you’re not getting enough through food alone, a supplement can help, but with attention to the right amount and form. Drinking plenty of fluids, moving your body most days, and balancing calcium with magnesium and vitamin D support healthy absorption and regularity.
Calcium citrate tetrahydrate brings real value for bone health when someone needs a supplement, but it asks for balance and attention. Trusted sources like the NIH and Mayo Clinic offer guidance to avoid unnecessary risks. Listen to your body, read the label, and don’t be shy about bringing your questions up at your next doctor’s visit.
| Names | |
| Preferred IUPAC name | Calcium 2-hydroxypropane-1,2,3-tricarboxylate tetrahydrate |
| Other names |
Calcium 2-hydroxypropane-1,2,3-tricarboxylate tetrahydrate Citric acid calcium salt tetrahydrate Calcium citrate-4H2O |
| Pronunciation | /ˈkælsiəm ˈsɪtreɪt ˌtɛtrəˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 5785-44-4 |
| Beilstein Reference | 2635084 |
| ChEBI | CHEBI:86156 |
| ChEMBL | CHEMBL1201563 |
| ChemSpider | 20642030 |
| DrugBank | DB11092 |
| ECHA InfoCard | 03b626ad-0da9-4b18-87fa-f2c5e9da751b |
| EC Number | E333 |
| Gmelin Reference | 1561396 |
| KEGG | C07442 |
| MeSH | D017602 |
| PubChem CID | 159393 |
| RTECS number | FF3525000 |
| UNII | 6T4MOS9S9F |
| UN number | Not regulated |
| Properties | |
| Chemical formula | Ca₃(C₆H₅O₇)₂·4H₂O |
| Molar mass | 498.43 g/mol |
| Appearance | White or almost white, crystalline powder |
| Odor | Odorless |
| Density | 1.9 g/cm³ |
| Solubility in water | 14 g/L (20 °C) |
| log P | -3.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.5 |
| Basicity (pKb) | 8.50 |
| Magnetic susceptibility (χ) | '−48.0×10⁻⁶ cm³/mol' |
| Refractive index (nD) | 1.52 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 330 J K⁻¹ mol⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2294.3 kJ/mol |
| Pharmacology | |
| ATC code | A12AA07 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. May cause skin irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Precautionary statements | Precautionary statements: P264, P270, P301+P312, P330 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD₅₀ Oral - Rat - 8,940 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50: 8,050 mg/kg |
| NIOSH | '' |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 1200 mg per day |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
Calcium citrate Calcium citrate malate Tricalcium citrate Citric acid Calcium carbonate |