Stories about rare earth elements often start in dusty chemistry labs from the 1800s. Cerium, named after the dwarf planet Ceres, popped up around 1803. Researchers soon realized cerium mixed well with other chemicals. The citrate salt form started drawing interest decades later as scientists looked for ways to harness cerium’s unique electronic structure, especially for catalysis and medical research. When industrial processes needed non-toxic, water-soluble cerium forms for animal feed, plant growth, and pharmaceuticals, cerium citrate jumped to the front.
Cerium citrate sits at a crossroads between medicinal chemistry and material science. This white to pale yellow powder or granular solid dissolves modestly in water and brings together rare earth metal properties with the chelating action of citrate ions. In practice, manufacturers usually offer cerium citrate with cerium in the +3 oxidation state. Major producers source raw cerium oxide from monazite or bastnäsite ores, using controlled reactions with citric acid to deliver a consistent final product aimed at reliability.
People who have handled cerium citrate will note its mild, earthy scent. The molecule’s structure: CeC6H5O7·xH2O. Its molecular weight hovers around 371.2 g/mol, depending on hydration. Water solubility ranges from moderate to fair, so slurry mixing requires patience. It tolerates air exposure well, avoiding quick oxidation. The compound does not burn or explode during standard storage, so big warehousing accidents almost never happen. Still, dust stays fine, so a spill quickly becomes a cleanup headache. Chemically, it balances between weak acidity from citrates and mild basicity from the cerium ion, which steers its reactions.
Every container should carry clear labels that detail both chemical and batch info. Labels identify the exact hydration level, purity—companies often aim for 98% or higher—plus lot number and any precautions. Certificate of analysis paperwork should break down heavy metal impurities, rare earth content, and water content. Barcode or QR code tracing brings transparency for quality checks or recall events. Producers include recommended storage conditions (dry, sealed, shade), shelf life, and manufacturer contact for rapid follow-up if an issue arises.
Traditional manufacturing involves dissolving high-purity cerium carbonate or oxide in dilute citric acid, stirring for hours at steady heat until ceasing precipitation. The solution gets filtered to remove unreacted solids, then dried at light vacuum or low-temperature ovens to form a free-flowing powder. Some labs prefer spray-drying for finer particles, useful in high-tech applications. Others test freeze-drying for ultra-pure pharmaceutical use, stripping solvents without aggressive heating. The critical step centers on controlling pH, since small shifts change purity and yield.
Cerium citrate reacts if mixed with strong acids, splitting into cerium salts plus free citric acid. Alkaline conditions push the cerium toward oxide or hydroxide forms—handy for catalysis or new material synthesis. In redox chemistry, cerium(III) in citrate form can shift up to cerium(IV), especially under heat or strong oxidizers. Some research explores blending cerium citrate with other chelating agents, like EDTA, to tweak reactivity or bioavailability. Pharmaceutical teams sometimes swap the citrate ion with tartrate or lactate to test effects on absorption, pushing the boundaries on what cerium compounds can do.
Cerium citrate doesn’t hide behind many aliases, but suppliers sometimes call it cerium(III) citrate, tricerium(3+) triscitrate, or just rare earth citrate. Common catalog numbers and CAS numbers provide quick reference for buyers. In animal nutrition circles, some distributors simply label it as “cerium feed additive.” These alternate names pop up in regulatory filings, research papers, and global shipping manifests. It pays to double-check product literature for the exact hydration state, which can throw off dosing or technical results if misread.
Workplace safety officers stress correct handling. Cerium citrate dust can cause irritation in eyes or, less often, skin. Inhalation, although uncommon, requires a mask and good ventilation. The jury’s still out on long-term occupational exposure, though animal studies signal low toxicity by oral route. Food and pharma regulations demand full traceability, heavy metal reporting, and clear input/output documentation for every batch. Spill protocols point toward vacuuming rather than sweeping—this keeps tiny particles from floating everywhere. Most countries align with GHS (Globally Harmonized System), which assigns pictograms depending on local toxicity assessments and usage context.
Demand for cerium citrate branches into a surprising mix of fields. Livestock producers mix it into feed to improve growth and immune responses, riding on evidence from controlled trials. Agricultural extensions experiment with foliar sprays to boost plant vigor in micronutrient-poor soils. Medical research highlights its use as a gentle antioxidant agent and in treatments for iron overload, since cerium competes with iron in biological pathways. Many environmental chemists look at cerium citrate’s catalytic properties when treating wastewater or degrading industrial dyes. Electronics labs blend it with polymers or glasses to test new optical materials. Each sector pushes suppliers to optimize purity, particle size, and solubility for its own needs.
Every year, cerium citrate shows up in more journal articles and patent filings. Recent studies in animal science focus on its ability to modulate microflora in the gut. Chemists try tuning the ce-citrate ratio to impact redox properties, aiming for sharper catalysts or improved imaging agents in medical diagnostics. Teams in green technology toy with cerium citrate as a replacement for toxic metal catalysts in industrial oxidation and combustion reactions. PhD students keep tinkering with solubility and stability, since shifting temperature or pH quickly changes performance and shelf life. Some work on combining cerium citrate with emerging nanomaterials, betting on enhanced activity and biocompatibility.
Published animal studies point to relatively low toxicity for cerium citrate, especially compared to heavier rare earths. Oral LD50 values hover at levels that make accidental exposure unlikely to cause harm in most settings. Extended use in feed shows either neutral or slightly positive health profiles, but gaps remain in long-term chronic exposure research, especially for workers or those managing manufacturing waste. Aquatic toxicity draws some attention, since cerium’s accumulation potential is nonzero and certain forms impact small aquatic life. Regulatory bodies keep reviewing new studies, sometimes tightening guidelines on disposal or spill cleanup near wetlands or rivers. The general scientific consensus ranks cerium citrate as safe when used with good lab and factory discipline.
The world’s growing hunger for sustainable materials keeps attention on compounds like cerium citrate. China and other rare earth heavyweights control much of the supply, which means future pricing and availability will track with mining, trade agreements, and demand for clean energy technologies. In green chemistry, cerium citrate’s role in low-impact catalysts stands tall as researchers try to sidestep chromium, mercury, or other old-school pollutants. Agro-tech circles anticipate more approved applications in plant health and animal feed, especially in regions looking to fine-tune after decades of fertilizer overuse. Pharmaceutical giants may fund deeper research into cerium-based drugs if early-stage results on antioxidant and metal chelation effects continue to impress toxicologists. The compound’s story looks far from finished—a testament to what careful chemistry and focused industry input can accomplish.
Most people probably haven’t heard about cerium citrate, unless they sit in a chemistry class or work inside a laboratory. Its name doesn’t spark much excitement outside of science circles. Yet, for anyone working with glass, electronics or even dealing with new forms of supplements, the compound keeps popping up. As someone who spent a little too much time fiddling with glass repair and mineral supplements, I’ve learned there’s more to cerium citrate than meets the eye.
Start with glass polishing. Watch someone restore an old watch face or repair a scratched phone screen. There’s a good chance those fine powders swirling around the glass include cerium citrate or its cousins. Cerium’s rare earth properties help polish glass at a microscopic level. The action smooths out scratches and leaves surfaces clear. This isn’t science fiction—the gleam in storefront windows, eyeglasses, or even high-end optics owes a bit to rare earth chemistry.
Cerium does not sit on the sidelines in electronics, either. Cerium compounds—including cerium citrate—find a seat in the production of certain types of capacitors. Capacitors act like small batteries; they store and release energy quickly. Researchers keep pushing to use rare earth compounds like cerium to make electronics work better under tough conditions.
The industrial world also finds room for cerium citrate. Factories sometimes rely on it as a catalyst. A catalyst isn’t consumed during its job but helps chemical reactions run smoother and faster. For those running big operations—whether making chemicals, processing metals, or handling wastewater—a boost from a good catalyst can mean less waste and more efficient output. Getting more done with less mess matters in real life, where nobody wants pollution or excess costs.
Lately, cerium citrate keeps turning up in dietary supplements. Some companies sell it as part of a mix designed to support metabolism, bone health, or help fight oxidative stress. Cerium itself isn’t an essential element for the human body, and health claims need scrutiny. I always go back to what I learned in pharmacology: rare earth elements shouldn’t go into the body without good evidence and sound scientific backing. People deserve to know what goes into pills and powders. Without long-term studies and clear safety profiles, tossing cerium compounds into supplements looks careless.
Cerium citrate shows how much rare earth elements influence modern life—often quietly, never with big headlines. These applications aren’t just academic trivia. Glass repairs, better electronics, and smarter factories all ripple through the lives of regular people. At the same time, every new use comes with the need for independent verification, tighter safety checks, and responsible sourcing. The world already knows the headaches that come from letting chemical shortcuts go unchecked.
Making the best use of cerium citrate isn’t just about chemistry; it’s about ethics and accountability. Regulators, researchers, and companies must stay transparent with new materials. Consumers—whether polishing a window or considering a new supplement—deserve straight talk, backed by evidence, not just marketing.
Cerium citrate caught people’s attention through claims popping up in supplements and online shops. Some tout it as a mineral boost, others see it as a curiosity from the world of rare earth chemistry. Folks who check labels carefully know the list of ingredients in any supplement matters, especially when the compound in question sounds more like something from a science lab than a dinner plate.
Cerium forms part of the so-called rare earth elements, mined in places like China and some parts of the U.S. In chemistry class, cerium sits with other lanthanides and rarely turns up in foods or daily routines. Cerium citrate is made when cerium teams up with citric acid, a compound you usually link to oranges, lemons, and diet sodas. Producers often use this product in industrial settings and as a lab reagent. The growing chatter about using cerium citrate in health supplements signals a push beyond traditional applications.
Looking at research from peer-reviewed journals and reliable chemical safety databases, evidence around cerium citrate’s effects in humans remains thin. Toxicology resources, such as the U.S. National Institutes of Health’s PubChem database, catalogue cerium compounds and cite them as low in acute toxicity based on animal tests. No strong consensus exists for long-term effects or cumulative toxicity in humans. The Environmental Protection Agency in the U.S. and international food safety authorities like EFSA haven’t cleared cerium citrate for food use or dietary supplementation.
I’ve read countless supplement labels and always cross-check any ingredient that doesn’t look familiar. Many trace minerals, like iron or zinc, have a clear track record and decades of nutritional data behind them. Cerium, in contrast, doesn’t belong on essential elements lists in most nutrition textbooks. Health authorities don’t set recommended daily limits for cerium because there isn’t enough proof the body uses it for any function.
Accidental industrial exposure to cerium compounds happens in certain workplaces, mainly through dust inhalation. Chronic occupational studies report some respiratory troubles among those with frequent high-level contact. Ingestion studies trail far behind. No big, controlled studies follow people using cerium citrate over months or years.
Consumers can trust known bodies like the FDA, EFSA, or Health Canada for safety rulings. As of now, cerium citrate doesn’t have approval as a dietary ingredient or food additive. Health professionals lean toward caution until clear, peer-reviewed evidence supports benefits and absence of harm.
People interested in rare minerals for wellness should ask their doctor before trying anything unfamiliar. If you want micronutrients, nature already provides plenty in fruits, vegetables, and balanced diets. Investing in exotic minerals or following trends online often carries more risk than reward when regulators and mainstream science haven’t caught up.
A better route to health ties in with getting routine nutrients from whole foods, checking for credible third-party testing in any supplement, and looking for trustworthy seals of approval. Always ask questions about unusual or untested ingredients, and search for honest, non-sponsored reviews.
Most people usually don’t come across cerium citrate in daily life. This compound pops up more around chemistry labs or specialty manufacturing. If you’re reading this, you care about understanding it for a reason—maybe research, maybe a unique health pursuit, maybe curiosity. I’ve looked through scientific journals, industry reports, and supplement claims: reliable info about proper dosing is tough to find. That alone should ring some bells.
Cerium itself is a rare earth element. Cerium citrate isn’t a dietary staple, nor a typical supplement suggested in clinics. The National Institutes of Health doesn’t list any standard nutritional guidelines. Most human research focuses on cerium’s industrial uses—think glass polishing, electronics, chemical processes. Few health professionals will even mention it, outside of lab safety protocols or material safety data sheets.
People sometimes talk about rare minerals as miracle ingredients or next-generation supplements. Cerium falls into that “cutting-edge, not enough evidence” category. What’s lacking above everything else is human clinical data. Top scientific reviews make it clear: no widely accepted recommended daily values exist.
Cerium doesn’t act like essential minerals. Our bodies need iron, calcium, magnesium, all backed by years of nutritional science. Cerium can interfere with enzymes and cell signaling when exposure rises above naturally encountered levels. One animal study in “Environmental Health Perspectives” (2012) raised some red flags: rats exposed to high cerium doses over weeks showed altered organ health and stress markers. The World Health Organization classifies cerium compounds as having low acute toxicity, but that doesn’t mean they’re harmless at any dose.
Suppose someone does find a supplement online or in a store that claims to contain cerium citrate. Sometimes the suggested dose is 10 mg daily, or maybe even less. Nobody can point to long-term safety data showing that’s a good idea. Nobody has mapped out how the body processes and clears cerium citrate across weeks or months. Kidney and liver function matter, as those organs try to remove trace metals. People with underlying health conditions face more uncertainty.
For me, the core lesson is simple: stick to compounds with deep, peer-reviewed evidence. Trust health professionals who rely on sound, published data. Stay wary of internet products selling quick fixes or rare compounds with little safety data behind them.
If you’re part of a research team or working inside a lab, cerium citrate dosage gets set by goals, protocols, and strict regulations. Look to Material Safety Data Sheets and follow industrial hygiene standards. If questions come up about unregulated supplements, speak with a toxicologist or a medical professional—not a random web forum.
Knowledge matters when stepping into less-charted territory like cerium citrate. Without robust studies, nobody can recommend a standard dosage for personal use. Science takes time, and with rare compounds, that means patience and skepticism serve your health far better than hype. Always look for evidence. That habit is worth more than any supplement claim.
Curiosity around cerium citrate comes from its growing presence in specialty industrial and medical products. It’s not something most people talk about at dinner, but folks who work around rare earth elements or those looking at new supplements may run across it. So, does it come with a cost to health? That deserves a clear-eyed look.
Cerium is a rare earth element, but that word “rare” can mislead. It’s more common than lead or tin in the earth’s crust. Researchers use cerium compounds such as cerium oxide for industrial polishing, auto exhaust systems, and research into antioxidant therapies. Cerium citrate isn’t as common in mainstream supplements or food products right now, but scientists keep poking at uses.
Very little hard clinical research exists on cerium citrate and human health. The FDA hasn’t approved it for medical use—so there’s not a government-backed safety profile or set daily limit. An Australian government chemicals assessment in 2014 mentioned cerium compounds may cause mild eye or skin irritation in raw form, though reactions depend on the specific chemical version and use context. That’s a cue for folks who handle powders or solutions in labs.
Direct reports from human use remain rare. A couple of animal studies suggest that large exposures could provoke mild digestive upset or changes to liver enzymes. One study on rats suggested possible shifts in kidney function at very high doses, but that’s not the same as what a worker or patient might encounter.
A lot of personal care and health products still don’t contain cerium citrate. Industrial workers might inhale or get dust on their skin. People with regular exposure sometimes report skin dryness, redness, or eye irritation, similar to many metal dusts or lab chemicals. If someone tries a product listing this ingredient and notices a rash or feels unwell, a doctor’s input helps make sense of things.
Sometimes marketing for rare minerals will speculate on benefits without matching research. Antioxidant abilities sound promising on paper. Actual evidence in people, though, stays slim, and benefits can’t be claimed without better trials.
Trust gets built on real evidence. Without it, there’s risk of side effects sneaking up because consumers don’t have clear facts. In my own work, I’ve noticed that a product’s track record over years matters—a newcomer warrants extra caution until reports pile up. Doctors, toxicologists, and chemists tend to nudge people back toward established supplements unless there’s a proven reason to go exotic.
People working in labs or factories benefit from protective gloves, goggles, and good ventilation to limit skin or lung exposure to cerium compounds. Washing up after handling chemical powders helps, too. Anyone thinking about adding supplements with rare earth elements should talk openly with a healthcare professional. Folks with kidney or liver conditions may want to skip experimental substances altogether unless guided by an expert.
Relying on reputable sources and published research makes sense. Companies willing to disclose detailed safety information and batch testing give some reassurance. If a product sounds too good to be true or skips over clear health warnings, it’s worth asking more questions.
Cerium citrate pops up quite a bit in labs and industries, celebrated for its role in catalysis, polishing, and more experimental uses. It’s a white powder that doesn’t scream hazard at first glance, but ignoring its quirks can lead to headaches.
Chemicals like this one deserve respect. Moisture, light, and air—those elements we take for granted—tend to mess with purity and performance. Over a few years working closely with chemical stocks, I learned the little habits that keep things clean and safe truly do make or break a lab’s workflow.
Cerium citrate reacts with damp air over time. That means even a quick dip into an open bag can introduce just enough moisture for subtle changes. Powder clumps, then the composition drifts. Performance slides slowly, which nobody wants in a serious application.
Glass or high-grade plastic bottles with solid, tightly screwed caps always offer better protection. Resealable bags don’t cut it for anything long term. If the bottle has a bit of silica gel to soak up stray humidity—better yet.
On my own shelves, anything that draws moisture, even nitrates or old salts, gets similar treatment. That discipline saves time and cuts expenses from ruined stock.
Cerium citrate prefers steady, cool temperatures. Room temperature works if air conditioning compares to what you'd expect in a climate-controlled storage area—steady and moderate. Storing this compound by a window or near heat sources invites breakdown. I’ve seen containers discolor or cake simply from being left near a sunny spot in spring.
A specialized chemical storage cabinet does most of the heavy lifting. Nothing fancy, just one that avoids big swings in heat or cold. Sometimes shared fridges come up in conversation, but condensation from opening doors usually causes more harm than good.
Direct light speeds up degradation of many chemicals, cerium compounds included. Simple cardboard or an opaque storage box removes that risk. Not much effort, but the return is fewer surprises during future testing.
Every time someone dips an unclean scoop or spatula into the jar, contamination creeps in. Set one clean scoop aside just for cerium citrate. This small ritual prevents cross-contamination, which creeps up fast in busy workspaces.
Label jars with the opening date and source. Over years, tracking down the who-what-when behind a batch gets tougher. Strong labeling means less confusion if standards change or a recall happens. My worst stock room moments always rooted back to vague or missing labels.
Cerium citrate likes a straight-forward, careful touch. With solid packaging, attention to moisture, a cool resting place, and strong habits, this chemical works as advertised and waste stays low.
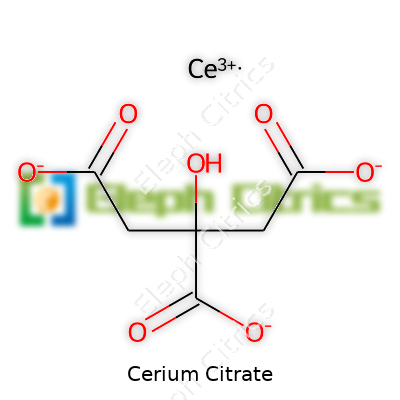
| Names | |
| Preferred IUPAC name | cerium(3+) 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Cerium(III) citrate Cerous citrate |
| Pronunciation | /ˈsɪəriəm ˈsɪtrət/ |
| Identifiers | |
| CAS Number | [4485-56-5] |
| Beilstein Reference | 157863 |
| ChEBI | CHEBI:131777 |
| ChEMBL | CHEMBL4298812 |
| ChemSpider | 21569629 |
| DrugBank | DB15974 |
| ECHA InfoCard | ECHA InfoCard: 100.122.866 |
| EC Number | cerium citrate" does not have an assigned EC Number |
| Gmelin Reference | 107168 |
| KEGG | C02712 |
| MeSH | D058125 |
| PubChem CID | 167607 |
| RTECS number | GU7387000 |
| UNII | 7FO2U8A3SQ |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C12H10Ce3O14 |
| Molar mass | 816.54 g/mol |
| Appearance | white powder |
| Odor | Odorless |
| Density | 2.2 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -4.3 |
| Acidity (pKa) | 6.4 |
| Basicity (pKb) | 8.7 |
| Magnetic susceptibility (χ) | -72.7e-6 cm³/mol |
| Refractive index (nD) | 1.52 |
| Dipole moment | 1.72 D |
| Thermochemistry | |
| Std enthalpy of formation (ΔfH⦵298) | -1765.5 kJ/mol |
| Pharmacology | |
| ATC code | A12CX |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264; P270; P273; P301+P312; P305+P351+P338; P330; P501 |
| Lethal dose or concentration | LD50 oral (rat) > 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50 > 5,000 mg/kg |
| NIOSH | No NIOSH. |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 2000 mg |
| Related compounds | |
| Related compounds |
Cerium(III) nitrate Cerium(III) chloride Cerium(IV) oxide Cerium sulfate |