Citric acid came into the limelight over two centuries ago, after a Swedish chemist managed to isolate it from lemon juice. Back then, the discovery sparked some curiosity, though its applications remained quite narrow. Once industries learned to produce it from black mold and sugar instead of squeezing tons of citrus fruits, the scene changed. That shift happened near the end of World War I, when advancements in fermentation and better understanding of microbial reactions helped factories turn out citric acid by the ton. That move also broke the bottleneck that made supply all too dependent on oranges and lemons. Scientists like James Currie transformed simple sugar into a multi-purpose, affordable chemical. Food, cosmetics, and health care industries opened their doors wide to this sour-tasting ingredient that started its journey as nature’s preservative in sour fruits.
Citric acid monohydrate looks like a white or slightly translucent powder, often with a crystal structure. No one walking past it on an industry floor could tell by sight alone how much work hides behind those grains. One big reason factories like it so much comes down to its water content—the “monohydrate” means every molecule brings some water along. That makes the product more stable and manageable for bulk customers. The ingredient finds its way into sodas, fruit juices, detergent tablets, bath bombs, even metal cleaners. Over the decades, suppliers have found ways to push purity up above 99.5%, and that means the same material sold to bakers can also fit strict pharmaceutical standards, provided labels and handling stay sharp in the supply chain.
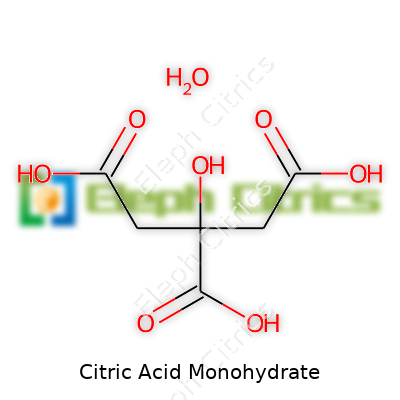
At room temperature, citric acid monohydrate takes the form of colorless crystals, with a molecular formula of C6H8O7·H2O and a molecular weight just above 210 grams. Its melting point hits around 100°C, at which time it sheds water and turns into anhydrous citric acid. The distinctive sour flavor comes from its three carboxylic acid groups, which also lend strong acidity to water solutions. The pH tends to stay between 2 and 3 in solution, making it a reliable acidulant in foods and beverages. Solubility in water is high, an important detail for beverage manufacturers. Once humidity rises, the powder can clump, so packaging matters a lot. Reactivity depends on partners in the mix: base substances neutralize it, turning it into citrate salts, while oxidizing agents will break it down into smaller bits, including carbon dioxide and water.
Most commercial batches display purity levels above 99.5% by weight, with strict control over heavy metals, sulfates, calcium, and other minerals. Packing details show lot number, net weight, country of origin, storage instructions, and shelf life. In the global market, the label often includes a chemical abstract service (CAS) number, an E number (E330 for citric acid), HACCP or GMP compliance marks, and allergen status. A key part of labeling comes from meeting regulatory needs—such as the US Food Chemicals Codex, the European Pharmacopeia, or local food safety agencies—so manufacturers adjust labels depending on where the material ships.
While the early pioneers squeezed it directly from citrus fruits, modern makers choose the fermentation route. Black mold species like Aspergillus niger feed on molasses or glucose extracted from corn or beet sugar. In large tanks, mycelium grows thick, and after a few days the broth fills up with citric acid. Filtering and precipitation bring out the acid, followed by thorough crystallization. Washing and drying steps produce a sparkling white powder with only small traces left over from the fermenting organism or feedstock. This approach avoids the wild price swings that came from relying solely on fruit harvests, and lets any part of the world—no matter how far from citrus orchards—get a reliable supply.
With three carboxyl groups and one hydroxyl group in each molecule, citric acid reacts in multiple directions. When mixed with reactive metals, it forms soluble citrate complexes, a trick exploited both as a food additive and in cleaning products that lift limescale. Neutralizing citric acid with bases like sodium bicarbonate creates citrates, often found in medical pills or as water softeners. In hot conditions with strong oxidizers, it breaks down into simpler compounds, which can help scrub away stubborn stains or keep industrial equipment free from mineral buildup. Sometimes, chemists modify the structure to create esters or ethers, unlocking new uses as plasticizers or surfactants.
On shipments and paperwork, citric acid monohydrate answers to several names. Chemists may write it as 2-hydroxy-1,2,3-propanetricarboxylic acid, β-hydroxytricarballylic acid, or just E330. Trade names can include food grade citric acid, Sour Salt, or acidulent 77, depending on traditions in different markets. Pharmacies may offer it under chemical codes, but shoppers buying SCRATCH-resistant cleaners or “natural” flavor drops might not even notice citric acid hiding on the ingredient list.
Few chemicals on the market have passed more safety screens than citric acid. Still, the powder can irritate eyes or the lining of the nose when handled without care, and inhaling a heavy dust cloud could make work uncomfortable for staff. Food regulators enforce maximum allowable limits in drinks and processed foods. On-site, manufacturers and warehouses stick to personal protective equipment, well-sealed packaging, and regular air monitoring. Like any acid, it shouldn't come into direct, careless contact with skin or metal food prep surfaces. Safe disposal depends on local water management practices, since run-off reaching rivers in massive doses could disrupt natural acidity levels. The global food trade strictly demands traceability, so suppliers document every batch from fermentation vessel to finished bag.
Every trip to the grocery store brings you face to face with citric acid. Soda cans, fruit-flavored candies, shelf-stable sauces, jams, jellies, and yogurts—all make use of its powerful tartness, pH control, and preservative properties. Behind the scenes in industrial kitchens, the acid balances flavors and keeps bacteria at bay. Cleaning product companies capture its limescale-busting powers in dishwasher tablets or sprays designed to leave chrome sparkling. Water treatment plants soften their output using citrate salts, which control mineral content. Pharmaceuticals turn to citric acid or its derivatives as acidulants and chelators—especially in antacids or energy powders. Even the cosmetic world borrows its exfoliating touch in skin peels or pH-adjusted shampoos. This kind of versatility keeps demand strong across the board, from food trucks and microbreweries all the way up to global manufacturing plants.
R&D labs take citric acid monohydrate for granted as a building block. Scientists tweak fermentation methods, hunting for new sources of feedstock like agricultural waste, aiming for sustainable, low-waste manufacturing. Pharmaceutical research leans into citrates for drug solubility and stability, hoping to make medicines more effective or taste less bitter. Biodegradable plastics and novel coatings attract green-minded researchers, who search for ways to swap out older, polluting chemicals. Analytical chemists use the acid for calibration and cleaning instrument surfaces between sensitive readings. The challenge now lies less in basic supply, and more in tuning each process for minimal waste, renewable inputs, and tight environmental controls.
Out of the hundreds of food additives in play, citric acid lands among the safest. Research on rodents and other test animals shows little evidence for chronic harm, even at doses much higher than typical human consumption. For people, excessive intake might upset stomachs or wear down dental enamel, though the amounts in fruits and processed snacks seldom reach those levels. A handful of sensitive individuals might react to residues from the fermentation process, but not to the acid itself. Food safety authorities around the world still review new batches for possible contaminants or byproducts, especially when manufacturers use feedstock from unexpected sources. So far, the consensus keeps citric acid monohydrate far from anything resembling a hazardous substance, compared to many food preservatives on the market.
Plenty of chemists, biologists, and engineers view citric acid monohydrate as more than just a sour note in food production. With demand in developing markets rising and the global shift toward green chemistry, its reputation for low toxicity gives it a head start. Industrial buyers look for new uses: scaling up metal cleaning, advancing eco-friendly packaging, or inventing better biodegradable plastics. As companies face tighter environmental rules, the ability to swap out harsh synthetics for milder, plant-based acids could open new market segments. People in R&D see opportunity in tweaking fermentation feedstocks, reducing water use, or recycling byproducts, helping citric acid shed even more of its carbon footprint. If sustainable chemistry holds the next wave of innovation, citric acid monohydrate appears set to stick around, quietly flavoring, cleaning, and preserving for generations yet to come.
Anyone checking food labels usually stumbles across “citric acid monohydrate” sooner or later. I’ve spotted it on everything from lemon-flavored candies to powdered drinks and even my laundry detergent. At first, it seemed like another one of those mysterious additives, but learning what it actually does brought a new respect for this unassuming ingredient.
Take a stroll through any grocery store, and there’s a good chance some product in your cart contains citric acid monohydrate. It keeps flavors bright and prevents the growth of mold and bacteria. No surprise, really, since fresh lemon juice has been used as a kitchen disinfectant for generations. Citric acid, pulled from citrus fruit or manufactured through fermentation, stops cut apples or ready-to-drink beverages from turning brown or going sour before their time. It's seldom the only preservative, but often it’s the unsung hero holding everything together.
In my kitchen, I've used citric acid to tweak the acidity in jams and jellies. A touch of tartness brings out the best in many fruits and gives that signature zing to sour candies. Even homebrewers drop it into wine must, tuning the taste to just the right level.
Citric acid monohydrate slips quietly into my cleaning routine. A scoop tossed into the dishwasher scrubs away limescale better than most name-brand options. My washing machine’s instruction manual even lists it as a safe way to descale the drum. Some people swear by it for clearing hard water marks from kettles and coffee machines. Its gentle but effective approach makes it popular among folks aiming to cut down on harsh chemicals at home.
The pharmaceutical world loves citric acid monohydrate—my own medicine cabinet has more than one product with its name on the back. Effervescent vitamin tablets fizz thanks to it. Chewable antacids rely on the acid to neutralize stomach problems. It even helps some medicines dissolve properly or keeps them stable on the pharmacy shelf. Dentists know it as a plaque buster or for prepping teeth before other treatments.
Being everywhere has its own challenges. Some parents worry about too much citric acid in snacks for kids, especially those who deal with sensitive stomachs. Rare, but some folks can get mouth ulcers if they go overboard on sour candies. Science backs up its safety in regulated amounts. Europe’s food safety authority and the US FDA both give citric acid monohydrate a thumbs up for food and everyday use.
Problems start with too much of anything. With citric acid, manufacturers hold the power to ease up where possible. Even at home, folks can keep it in the cleaning caddy and medicine cabinet, but moderation always pays off.
Some clever minds are working to produce citric acid using less energy or swapping out old fermentation tech for greener options, aiming to make each batch more eco-friendly. There’s value in pushing for clear labeling, better guidelines on safe use, and more public info about what’s in our favorite foods and products. Familiar faces in our pantry deserve that bit of attention, and citric acid monohydrate is no exception.
Citric acid monohydrate sounds like something you’d spot in a high school chemistry class, but it’s actually a regular visitor to most people’s pantries. Found in everything from lemonades and sodas to salad dressings, this sour-tasting compound sharpens flavors and helps foods last longer. My own kitchen always has at least one box of lemonade powder, with citric acid nestled high on the ingredient list. Plenty of people never give it a second thought before pouring a cold glass.
The reason citric acid pops up so often: it’s cheap to make and does its job well. It started off as something mainly squeezed from lemons and limes, but these days, manufacturers tend to rely on a fermentation process using a mold called Aspergillus niger. This practice has been the standard for decades and it’s how most commercially available citric acid is made now. The monohydrate part just means it contains water, which helps with handling and blending.
There’s always a reason to dig deeper, especially since food additives can sometimes carry hidden baggage. Most health authorities, including the FDA and other major global agencies, have greenlighted citric acid monohydrate as a safe ingredient when eaten in normal amounts. This isn’t just for adults—kids and older folks fall under the same umbrella. I’ve eaten my share of tangy candies and drunk more than enough soft drinks, and nothing out of the ordinary has ever come from the citric acid in them.
Scientific reviews back up what most of us experience. Research has shown that the body breaks down citric acid without storing or accumulating it. This makes sense because your body actually relies on citric acid naturally, running it through the famous Krebs cycle (something I remember from high school biology), which turns sugars into energy.
It’s tough to find real-world reports of problems linked to eating citric acid monohydrate in food-level quantities. Tiny groups of people, often those who already have certain allergies or inflamed guts, can experience irritation or reactions. Some folks claim to feel effects from processed foods that contain citric acid, but it’s hard to pin down citric acid as the main cause when lots of other ingredients could play a role. In my circle, I’ve never heard someone blame a stomach issue directly on this compound.
One thing nobody can ignore: additives pop up everywhere. It’s not just about one element—it’s about the full picture of what lands in the shopping cart. Someone eating a diet full of processed foods grabs much more citric acid than someone who cooks at home from scratch. Most people could do with a little less of the ultra-processed stuff, not just because of citric acid, but for broader health reasons.
Rather than picking up too much suspicion about a single ingredient, putting a focus on simple, whole foods sets a person up for better health. It helps to keep an eye on food labels, learn where ingredients come from, and balance out convenience with home-cooked meals. No one product is going to make or break a diet, but being mindful about everything on your plate always pays off. Citric acid monohydrate has staked out a spot in modern kitchens and factories alike, and as science stands today, it looks like a safe bet as long as moderation and a bit of label-reading guide the way.
Citric acid monohydrate pops up almost everywhere—from kitchens to factories, in cleaning supplies, even inside vitamin bottles. People often forget that, despite its harmless appearance, sloppy storage can mess up both its quality and its purpose. I’ve seen bags left open on warehouse floors transform into hard blocks of useless powder after just a few humid days. There’s plenty at stake if citric acid picks up moisture or impurities from its surroundings.
Humidity loves citric acid. Left out in a damp environment, the powder clumps and can attract mold. In some spots, summers bring out the worst—you’ll return after a weekend to chunks, not grains. Moisture in the package causes it to degrade quicker. Imagine noticing crystals have yellowed or the bag gives off an odd odor. That means it’s time to throw out what could have been used, and spend more on restocking.
The effect isn’t just visual. The acid reacts with what’s in the air, especially if you store it near cleaning chemicals or in kitchens where strong odors float around. Citric acid that soaks up strange smells isn’t something you want to use in food or drinks.
A lot of us just throw it in any cabinet or back room, thinking, “It’s powder. What’s the worst that could happen?” Over the years, though, I’ve learned to save money and avoid headaches by following a few tried-and-true habits. One bakery I worked with stored citric acid in loosely tied plastic sacks next to the dishwasher. Not a smart move. Those sacks absorbed steam all day, so the owner had to toss the whole batch once little black dots appeared. Since then, they moved it up high and into a tightly sealed container. Zero waste since then.
At home, keeping citric acid in glass jars with a gasket lid makes it easy to scoop out just what you need, without exposing the rest. No one likes lumps in homemade lemonade. Dry, dark, and cool spots (like pantry shelves above the ground) work much better than above the stove or under the sink where pipes sweat. It’s about reducing contact with humidity and heat—two things that change citric acid for the worse.
Simple habits can stretch a package for months, even a year. Always keep the packaging sealed tight. If you buy in bulk, split the bag with close friends and store your share in smaller airtight containers. That way, you don’t keep opening a huge package for every small use.
For commercial kitchens or workshops, large bins with snap lids keep the contents safe. Place desiccant packs inside if humidity becomes a problem—these small packets suck up stray moisture and keep things dry inside. Remember to check the expiration date now and then; keeping old, half-caked powder isn’t worth the risk.
Careless storage doesn’t just cost money. It can mess up the results of recipes, destroy food safety, and even trigger health concerns if the acid goes bad. Schools and factories handle much bigger volumes, so a simple slip-up can spoil hundreds of pounds. For scientists, degraded citric acid ruins results. For cooks, it just means bad flavor. Either way, the fix boils down to being a bit more careful with storage—something worth thinking about each time you reach for that white powder.
In the world of food and household chemicals, citric acid keeps showing up on ingredient lists. It pops up in fizzy drinks, canned tomatoes, cleaning powders, bath bombs—you name it. But “citric acid monohydrate” and “anhydrous citric acid” are both listed on product labels, and to most people these names sound like sci-fi jargon. I used to reach for whichever was cheaper on the shelf until a food chemist friend explained the reason for the difference, and why it actually matters sometimes.
Citric acid monohydrate contains water woven into its crystal structure. If you picture crushed ice, it sort of makes sense—water locked together with the acid molecules. The “monohydrate” tag means every molecule of citric acid in the powder comes with an extra “watering can” attached. Anhydrous citric acid skips the water. The crystals are just acid. If you look closely, the monohydrate version feels a bit more clumpy, sometimes forming small lumps on humid days. It dissolves quickly but can feel stickier to the touch.
It’s easy to think this doesn’t matter much. Honestly, it depends on how someone uses the powder. In food processing, precision matters. If you run a recipe needing a very specific sour hit—maybe in hard candies or carbonated drinks—a chemist wants to account for that little bit of extra water in the monohydrate form. On a large scale, even half a percent off can ruin a batch. Using the anhydrous version means the recipe is a little simpler: pure acid, predictable result, and no calculation around added water.
Kitchens get humid, pantries get warm. Anhydrous citric acid handles moisture a bit better than monohydrate. The monohydrate version, with its built-in water, loves clumping up in humid weather, and sometimes that turns storage containers into a sticky mess. I’ve dealt with sticky lumps that refuse to dissolve until you smash them with a heavy spoon. The anhydrous form definitely powders more easily, which makes it popular among people making their own bath products or using it as a cleaner.
If someone is just squeezing lemon flavor into drinks or looking for a sour fizz in home recipes, both types work. I’ve swapped them out in jellies and didn’t notice much difference in taste. The flavor is the same, but the sourness can feel a bit more intense with anhydrous citric acid since you get a bit more acid per spoonful. If you’re following a recipe for home canning that calls for citric acid, either form can work but it’s smart to keep the water content in mind—or just follow the instructions and measure carefully. If you see clumping, try drying out the powder with a little time in the oven (on a low temp), a trick that saved me more than once.
Some people stress about purity and label differences, but unless you’re running a lab or a food factory, both citric acid forms will keep tomatoes safe in jars and bathrooms sparkling clean. The important thing is storing it dry, measuring with a steady hand, and knowing that, in the end, both do the job of adding a tart snap where you want it. That’s what matters in any home kitchen or workshop.
Citric acid monohydrate shows up in so many kitchens, cleaning cabinets, and food factories. People tuck it away in plastic jars or forgotten pantry corners. On every package, a stamped date promises a future. This “best by” date usually points to 3-5 years from production—but that number comes from cautious habits, not wild chemical breakdown.
Humidity creeps in as the main culprit. Citric acid monohydrate absorbs moisture from air faster than you might expect. Even thick plastic lids can let in enough water vapor over time to clump up the powder. Once damp, it hardens, cakes, and sometimes shifts from crystal powder to a sticky mess.
Heat and light don’t ruin citric acid as quickly as they destroy some vitamins, but warmth will speed up water absorption and may eventually encourage breakdown—particularly if the container isn’t tightly sealed. People often ignore the basics of keeping pantry goods in cool, dry places, treating acids like sugar or flour, only to be surprised later by unexpected lumps.
If you store it in a sealed, dry container, citric acid monohydrate outlasts its labeled shelf life and can work just fine even after five years. In my own kitchen, I’ve tested a scoop from a six-year-old jar. It still dissolved clear in water, tasted like lemons, and cleaned kettles without residue. Science supports that stability: studies show little change unless moisture sneaks in.
Most citric acid monohydrate fails not because it “goes bad,” but because of contamination. Double dipping spoons, dirty hands, or wet measuring cups add unwanted guests to the jar. Mold or odd smells signal that it should take the trip to the trash.
Long shelf life helps consumers hold onto big bulk buys without worry. For food manufacturers, waste from expired ingredients drives up costs and product recalls. No one likes the taste of cleaning supplies gone stale, but real waste comes from assuming every date means instant spoilage.
Citric acid shows up in surprising places: preserving jams, boosting sourness in snacks, cleaning old kettles, chelating minerals in brewery lines. Losing potency or absorbing flavors means ruined batches and frustrated cooks. The cost of waste adds up—both for homes hunting value in big-box bags, and for businesses fighting for every penny.
Simple steps stretch the usable life. Tight-sealing containers, dry hands, and cool storage make the difference. I recommend transferring powders into thick jars and dropping in food-safe silica packets if the area’s humid.
Some companies go further by offering small, single-use pouches. These lower the risk of repeated exposure, particularly for people who only use a bit at a time. Most home users find that splitting up a big purchase into several airtight jars cuts risk.
The number stamped on the jar gives a guide, not a deadline. Citric acid monohydrate’s real shelf life often reaches well past the label as long as water stays out. If it clumps or smells off, swap it out. For people and businesses looking to save money or cut waste, smart storage lets citric acid do its job for years past the “official” date.
| Names | |
| Preferred IUPAC name | 2-hydroxypropane-1,2,3-tricarboxylic acid monohydrate |
| Other names |
2-Hydroxypropane-1,2,3-tricarboxylic acid monohydrate C6H8O7·H2O E330 Lemon salt Citronensäure Monohydrat Acidum Citricum Monohydricum |
| Pronunciation | /ˈsɪtrɪk ˈæsɪd ˌmɒnəʊˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 5949-29-1 |
| Beilstein Reference | 1714228 |
| ChEBI | CHEBI:1008 |
| ChEMBL | CHEMBL17753 |
| ChemSpider | 858 |
| DrugBank | DB04272 |
| ECHA InfoCard | 100.018.230 |
| EC Number | 201-069-1 |
| Gmelin Reference | 1262 |
| KEGG | C00158 |
| MeSH | D002244 |
| PubChem CID | 311 |
| RTECS number | GE7350000 |
| UNII | 2968PHW8QP |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID8046725 |
| Properties | |
| Chemical formula | C6H8O7·H2O |
| Molar mass | 210.14 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | Density: 1.542 g/cm³ |
| Solubility in water | 59.2 g/100 mL (25 °C) |
| log P | -1.72 |
| Vapor pressure | < 0.01 hPa (20 °C) |
| Acidity (pKa) | 2.2 (first), 4.3 (second), 5.6 (third) |
| Basicity (pKb) | pKb: 9.59 |
| Magnetic susceptibility (χ) | -51.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.334 |
| Dipole moment | 8.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 248.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2082.0 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2060 kJ/mol |
| Pharmacology | |
| ATC code | A09AB13 |
| Hazards | |
| Main hazards | Not a hazardous substance or mixture. |
| GHS labelling | GHS07, Warning, H315, H319, H335 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Autoignition temperature | 345 °C |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 Oral Rat: 5400 mg/kg |
| LD50 (median dose) | 5040 mg/kg (Rat, oral) |
| NIOSH | WH7500000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 3 mg/kg bw |
| Related compounds | |
| Related compounds |
Citric Acid Anhydrous Sodium Citrate Potassium Citrate Calcium Citrate Citric Acid-Sodium Citrate Buffer |