Copper has forged a deep-rooted path in human history, showing up as a trade good, an essential metal in wires, pipes, and so much more. After industries and researchers combed through every combination you could imagine, Copper Citrate landed on the map as a compound worth studying for both its practical uses and biological activity. Tracing its trail from the early days, scientists spent years pinning down stable salt forms of copper that worked in nutritional supplements and industrial processes. Copper Citrate’s place isn’t just a flash in the pan: its story connects the work of 20th-century nutritional chemists aiming to solve mineral deficiencies, lab techs testing safe absorption forms, and manufacturers creating safer, more effective copper additives. Nutritional experts and chemical engineers never stopped looking for ways to make copper easier to handle and less prone to causing harm during use, which led Copper Citrate to stand out among better-known copper salts.
Copper Citrate is a teal-blue powder with a slightly metallic, sour taste, known mostly as a bioavailable supplement and a reagent in the laboratory. This compound brings together copper, which bears the heavy responsibility of participating in many body systems, and citric acid, found at the heart of lemon juice and most fruit. Together, they form a stable ingredient that can stand up to heat, moisture, and a decent amount of mechanical stress. Practical folks in nutrition, agriculture, and even cosmetics choose this form of copper because it delivers trace minerals without the sharp bite typical copper salts bring along. In food fortification, supplement blends, and specialty farming, Copper Citrate gets the nod thanks to its balance between effectiveness and gentleness on the stomach.
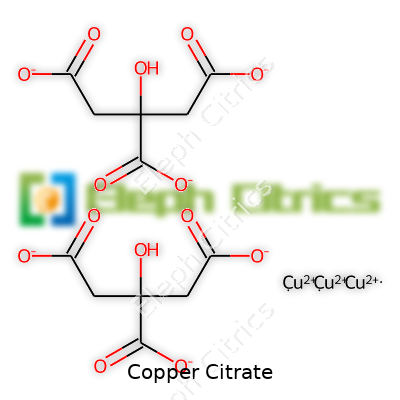
Copper Citrate stands out thanks to its distinct blue-green hue, a solid walk-away sign of copper’s presence. Its density and crystalline structure provide clues for lab techs and process operators. This solid resists clumping and can handle light moisture without sponging up too much water. It dissolves slowly in liquids, which testers use to their advantage when fine-tuning delivery in supplements or fertilizers. Copper Citrate doesn’t just sit there looking pretty—its formula, C6H5CuO7, reflects a well-defined composition that resists wild swings in acidity and basicity once dissolved. The copper ion in this form stays less reactive compared to copper sulfate, leading to a stable shelf-life under normal storage. With a moderate melting point and limited volatility, handling Copper Citrate doesn’t require the same precautions reserved for some nastier chemical cousins.
Being a specialty chemical and dietary ingredient, Copper Citrate falls under strict manufacturing protocols. The standard product comes in high-purity grades for pharmaceutical or food use, with minor residuals like moisture and trace metals tightly controlled. Quality specifications look for copper content in a narrow range, usually around 23–25%, to guarantee that a given batch will work across different settings. Labels carry not only the batch number and grade but the actual copper and citric acid content, since users build their formulas based on elemental copper. Many markets demand it include allergen statements and country-of-origin traceability. Testing labs report on mesh size, solubility, and particles-per-million for impurities like lead and arsenic—nobody wants hidden trouble sneaking into their process or product line.
Making Copper Citrate calls for a straightforward reaction between copper carbonate or copper oxide and citric acid, usually in warm water. The process needs careful attention to reaction rate; rushing the steps produces inconsistent yields and oddball byproducts. After mixing, a filtration catches undissolved bits, and evaporation or gentle heating helps crystals of Copper Citrate grow. On a bigger scale, automated vessels mix the reactants under constant agitation, pumping off excess liquid before casting or drying into powder. Production teams keep an eye on temperature and pH to ensure the copper doesn’t get left behind as unreacted residue. The beauty of this method is that it skips dangerous solvents, keeps waste manageable, and gives control over particle size and hydration. Small tweaks—like adding seed crystals or using high-purity water—let the processor fine-tune the batch for everything from research-grade to food-grade material.
Copper Citrate isn’t stuck in its original form. With a tweak to temperature or the pH of its surroundings, it switches from a dry salt to a more reactive complex, releasing copper ions into solution. In labs studying catalysis or biomedical effects, researchers sometimes pair Copper Citrate with reducing agents to form nanoparticles with potential medical or environmental uses. If you take Copper Citrate and run it through heat in a reducing atmosphere, you end up with metallic copper powder free of more aggressive acid leftovers, which proves handy in making advanced copper materials for electronics. Under the action of oxidizers or chelants, Copper Citrate breaks down to give up both its copper and organic acid, feeding into a variety of technical and research applications.
Chemicals seem to acquire a dozen names for every one official standard. Copper Citrate crops up in catalogs as copper(II) citrate, cupric citrate, or even simply “citric acid, copper salt.” Some product lines lean on a standardized code, like E-Cu Cit, in feed additives. It may also show up as “copper tris(citrate)” in technical references, reflecting the exact way the molecules hook together. Regardless of the alias, these all refer to a class of materials meant to deliver copper in a controlled, gentle fashion for biological or technical use.
Copper itself rides a fine line between necessity and toxicity. Copper Citrate, thanks partly to its natural acid partner, keeps risks lower compared to more caustic copper compounds. Workers handling this material use basic dust control and avoid skin contact, since high doses of copper can irritate the skin and mucous membranes. Facilities follow local rules covering storage—away from strong acids, strong bases, and reactive metals like aluminum. International standards, including those managed by food and supplement overseers like the FDA and EFSA, set maximum daily limits for copper in products, keeping levels well below anything that could trigger copper overload or liver issues. The label and data sheet spell out what to do in a spill or overdose case, and good operators maintain spill kits and respiratory masks for emergencies. Manufacturers invest in regular air sampling and staff training to cut occupational risk, learning from the hard lessons of the past.
Copper Citrate shines brightest where a measured, safe copper boost is needed. It gets the nod in functional foods and dietary supplements designed to top up copper for people at risk of deficiency. Farmers lean on it in specialty fertilizers and micronutrient blends to keep plants healthy and bolster resistance to fungal disease. Animal nutritionists select this form in feed premixes for livestock, betting on steady mineral uptake while dodging harsh impacts seen with more soluble copper salts. Some skincare lines add Copper Citrate for its mild antibacterial properties, betting on benefits without the sting linked to stronger copper chemicals. Lab techs treat Copper Citrate as a control standard or a precursor in nanoparticle work, benefiting from its predictable behavior and moderate reactivity.
There’s a big push in science to understand how different forms of copper interact inside living systems and how best to deliver them. Researchers weigh Copper Citrate against other copper salts and against organic-bound copper in both animal and human studies, asking tough questions about absorption, metabolism, and safety. Nutritionists chase answers about whether copper citrate truly gets absorbed more smoothly, noting slight bumps in bioavailability compared to other copper salts. Tech developers see Copper Citrate as one cog in building safer, smarter trace nutrient blends in crops and animal feed. Work continues on custom-tailored nanoparticles that use Copper Citrate as a precursor, hoping to unlock new directions in energy, medicine, and electronics. The steady march of trials, published data, and real-world case studies strengthens public trust and pushes companies to refine their product lines.
Copper Citrate, like any copper supplement, draws scrutiny about how much is safe before the benefits turn into hazards. Toxicology teams pinpoint the thresholds where copper stops helping and starts causing nausea, gastrointestinal upset, or worse—liver damage—especially in those with preexisting liver or metabolic conditions. Rodent studies and human trials both suggest Copper Citrate's copper gets absorbed at a moderate, steady pace, with safer margins than highly soluble copper sulfate. In animals, the safety window is relatively broad, but scientists note that individual health status, genetics, and diet—like high intake of vitamin C and zinc—may shift the balance. Extensive reviews by dieticians and regulatory panels give Copper Citrate a green light for controlled dietary use, as long as intake stays below established maximums: for most adults, under 10 milligrams of copper per day.
Copper Citrate’s story isn’t done. Demand for efficient mineral supplements, cleaner crop nutrition, and testable, traceable chemical building blocks in manufacturing continues to rise. With growing doubts about food quality and mineral depletion in soils, traceable, standardized forms of minerals like Copper Citrate will stick around in both food tech and agriculture. The green chemistry movement may also shine a brighter light on Copper Citrate, with companies searching for nontoxic, easily recoverable precursors in electronics and metalworking. Research into slow-release copper compounds and their impact on both humans and the environment is just heating up, with patents popping up for new uses every year. While the basic science stays grounded in decades of evidence, the real action will come from tech-driven refinements and the drive to tailor copper use safely in an ever-expanding set of fields.
The term "copper citrate" might sound like something cooked up in a chemist’s lab, but it plays a direct role in many parts of daily life. Whether you’re reading about it on a vitamin bottle or hearing your doctor mention it, copper citrate makes its mark because copper stands as one of the most essential micronutrients for the human body. I remember the first time a friend of mine, who runs a small organic farm, explained how copper fits into both soil health and animal diets. It helps enzymes work, fuels energy production, and builds strong connective tissue. Most folks don’t think of the copper in their homes as having much in common with their health, but there’s a direct link. If copper runs low, your body feels it: fatigue, weak bones, and a sluggish immune system aren’t far behind.
Anyone who’s spent time scanning the aisles for mineral supplements knows there’s always a debate: chelated copper, copper sulfate, copper gluconate, and then this one — copper citrate. The difference boils down to how well the body absorbs the mineral. I spoke once with a nutritionist who explained things pretty simply: citrate forms of minerals generally absorb better in the gut compared to others. That means copper citrate doesn’t just pass through you; it’s actually available for your body to use.
Copper plays a supporting role in forming red blood cells and keeping nerves healthy. In a world where vegetarian and vegan diets grow more common, copper deficiency emerges more often than people assume. Animal foods are some of the easiest sources of copper. Without them, it helps to have forms of copper that the body recognizes and pulls in easily. That’s why so many dietitians suggest copper citrate supplements to folks who might be missing out, including those who avoid meat or have trouble absorbing other minerals.
Supplements aren’t its only home. I learned from local food scientists how copper citrate finds its way into fortified foods and animal feeds. Copper proves crucial for livestock health: without enough, animals can suffer from stunted growth and reproductive problems. Citrate forms show up in animal feeds because they deliver copper reliably, making sure both pets and farm animals get what they need for healthy growth. Crops need copper for similar reasons, and deficiencies in soil can hit yields hard. By providing copper in a form plants and animals handle well, farmers can cut back on losses and waste — two things small producers can’t afford.
With all the benefits, it’s easy to forget too much copper can be a real problem. Years ago, I listened to a talk from a toxicologist who described copper overload symptoms that included stomach pain, nausea, even organ damage. That hammered home the point: supplements and fortified foods shouldn’t be taken blindly. The recommended daily allowance for adults runs about 900 micrograms. Lab tests can spot deficiencies or excesses, and a good doctor will check for other causes before suggesting a supplement. Public education matters here. Labels need clear dosing information, so nobody ends up with more copper than they need.
I see great value in research and education. More research could shed light on which groups actually benefit most from copper citrate. Healthcare professionals and teachers need resources for spreading the word safely, with balanced info and warnings about both shortage and excess. Farmers and food producers benefit from easy access to high-quality mineral sources. The basics remain clear: copper citrate helps people and animals thrive, but balance is always the key.
Copper plays a big part in how the body runs its daily business. Growing up, I remember my grandmother saying her water pipes “gave her minerals.” There’s a kernel of truth there, even if it’s not the safest delivery method. For a long time, copper has stood out because of its role in forming red blood cells, keeping nerves strong, and helping with iron absorption. Copper citrate, a type of supplement made by combining copper with citric acid, has started catching attention in health circles for being an easy-to-absorb form.
Copper works with iron to help the body produce red blood cells. Tiredness that doesn’t budge, feeling weak, or constant brain fog sometimes come from not getting enough copper. Studies from the National Institutes of Health show copper deficiency can lead to anemia—much like low iron. Copper citrate can help bump up your copper levels in a form the body takes in without much resistance.
On the immunity side, copper helps white blood cells hunt down invaders. Without enough, the immune response gets sluggish. Infections hang around longer. Research featured in the journal Frontiers in Immunology calls copper a “frontline defender” for immunity, supporting the natural clean-up crew that protects us from bacteria and viruses. Copper citrate supplements step in where diets fall short.
The brain relies on copper to build chemical messengers like dopamine and serotonin. Memory problems or mood swings can signal copper running low. In my years writing about nutrition and talking with health professionals, they often mention how proper copper levels keep the nervous system firing smoothly. Copper citrate brings another advantage—it generally causes less stomach trouble than copper oxide or sulfate.
Some research in Neurobiology of Aging connects healthy copper intake to slower mental decline in older folks. The risk of Alzheimer’s may go up if copper sinks too low, though too much copper can be just as rough. Taking a daily supplement with copper citrate, within recommended limits, gives the brain raw material it can actually use.
Bones need copper for strength. The mineral helps lay down collagen, giving bones and connective tissues their backbone. According to the Linus Pauling Institute, long-term copper deficiency links to weak bones and joint problems. In folks with athletic lifestyles or anyone at risk of osteoporosis, copper citrate can serve as added insurance for bone health.
Copper keeps the heart steady by helping blood vessels stay flexible and strong. The American Heart Association notes that not enough copper can stiffen the arteries, which sets the stage for high blood pressure. By sticking with a form like copper citrate, people often find better absorption and fewer digestive issues.
Everyone absorbs minerals differently. I’ve seen friends and clients who eat balanced meals but still show signs of copper deficiency—think brittle hair, sluggishness, frequent colds. Copper citrate suits those needing an extra boost or dealing with health issues that slow down mineral absorption, such as celiac disease or certain digestive disorders.
Doctors advise not to go overboard. Too much copper can cause trouble, from stomach pain to liver issues. Always check with a qualified health provider before starting supplements, and try to get nutrients from real food like nuts, beans, seafood, and leafy greens. Copper citrate works best as a top-off for those not getting enough—nothing more, nothing less.
Copper doesn’t get the same attention as zinc or magnesium, but it pulls plenty of weight inside the body. Red blood cells rely on it, nerves fire better with it, and the immune system thanks you when you get enough. Getting the right amount isn’t tough for most people, but some health conditions, restrictive diets, or absorption issues create a need for more.
Before anyone starts looking for a copper citrate bottle, it pays to know if you actually need it. Too much copper can affect the liver and upset your gut. Blood tests provide a clear answer, and a quick chat with a healthcare provider can help you figure out if there’s a real deficiency or if the problem lies somewhere else.
Copper citrate lands softer on the stomach with food, especially if you have a sensitive gut. Taking it with a meal helps prevent nausea, which copper supplements sometimes cause. People sometimes reach for it on an empty stomach, but food slows absorption just enough to keep things comfortable.
Most adults manage fine with around 0.9 mg of copper per day. Supplements come in different strengths. Some bottles offer much higher doses—these aren’t for everyone. Too much copper, taken daily, stacks up in the body over time. Sticking to the daily recommended amount (or what your doctor suggests) keeps things safe.
Zinc and copper battle for absorption in the gut. If you take a zinc supplement, hold off on copper for a few hours. Spacing them out lets your body grab what it needs from both. Taking too much zinc for too long can lead to lower copper levels, which shows up as fatigue, pale skin, or even trouble walking in some cases.
Supplements aren’t all the same. Go with a brand that lists the exact dose and doesn’t hide behind vague ingredient lists. Look for third-party testing—the seal of a big lab or certification organization helps. Glass bottles protect copper citrate from light and heat, which can degrade supplements over time.
Liver problems or conditions like Wilson’s disease mean copper builds up instead of being processed and flushed away. For folks with these issues, copper supplements cause more harm than good and sometimes create dangerous symptoms. Children and pregnant women also need a careful hand; their copper needs change fast, and too much brings its own set of problems.
Supplements help in a pinch, but food gives a steady, low dose every day. Shellfish, nuts, seeds, and even dark chocolate come packed with copper. Diet rich in these foods sends plenty of copper in a gentle, controlled way.
If you find yourself feeling queasy after taking copper citrate, switch to a different meal, try a smaller dose, or talk with your healthcare provider about a different form. If the current supplement leaves a metallic taste or seems hard on the stomach, look for chelated versions or switch to food-based options. Never ignore odd symptoms—joint pain, fatigue, or stomach problems—since they sometimes point to too much copper in the body.
Staying healthy isn’t just about adding another pill. It’s about listening to your body, checking in with professionals, and making small shifts based on real needs. One blood test, a smart supplement pick, and tuning in to your own reactions create the safest way to supplement copper citrate.
Copper finds its way into supplements for good reason: the body counts on it for healthy blood vessels, proper nerve function, and immune support. Copper citrate lands in the mix as an organic form of copper, easy for the gut to absorb. It usually turns up in supplements for folks who aren’t getting enough from their food or have certain health issues that drag copper down. Still, like anything you swallow for your health, copper citrate isn’t always a free ride—side effects can show up, especially if your system ends up with too much on board.
Most adults need only a tiny bit of copper each day—think about 900 micrograms. Fill up beyond that, and trouble starts. From what I’ve seen working with different supplement users and going through public health data, the signs of copper excess aren’t always loud. Some folks wake up with stomach cramps, nausea, or even diarrhea after upping their dose. In rare cases, vomiting and headaches hit hard enough to make someone call the doctor.
The National Institutes of Health stands by its upper limit: 10mg of copper a day for grown-ups. The reasoning is simple: more copper in the body means the chance for liver stress goes up. The liver stores and releases copper at its own pace, but overload it for long enough, and the system cracks. A few documented cases showed people landing in the hospital with serious liver issues, and blood labs confirmed sky-high copper.
Daily copper intake that stays too high for months or years creeps up on people. The early signs can fool you—just feeling tired, some joint aches, or the odd change in mood. Over time, high copper can mess with brain health, raising the risk for memory issues. Vitamin and mineral imbalance also sneaks in, since too much copper can wrestle with zinc for absorption.
From a personal angle, coaches and dieticians often spot athletes popping multiple supplements—multivitamins, “performance" mixes—all hiding copper somewhere on the back label. Stack enough sources and even healthy eaters tip past the limit. I’ve seen clients hit with regular stomach upset, blaming food intolerances, only to have blood tests point towards copper piling up instead.
Children face a greater risk from higher copper doses, just because their bodies can't process as much. People living with Wilson’s disease or other rare liver conditions must avoid copper supplements entirely, since their bodies can’t flush it out. For pregnant and breastfeeding women, extra caution is wise—talking with a doctor before adding copper citrate helps avoid surprises.
Testing tells the real story. Blood and urine workups reveal copper status. Doctors can catch a buildup early and offer changes in diet, swapping to copper-free supplements, or in some cases prescribing a chelator to pull copper out.
Copper citrate can support health where there’s a true shortage. But popping high doses in the pursuit of "optimal health," without tracking total intake, opens the door to issues most of us would rather skip. It's smart to double-check every label and chat with a healthcare provider, keeping the balance where it belongs. Supplements can fill a gap, but it never hurts to ask if you’re actually missing something before adding more.
Copper matters to the human body. You find it in nuts, shellfish, seeds, even dark chocolate. The mineral supports nerve health, helps produce red blood cells, and keeps our immune systems running. Copper citrate appears on the supplement shelf as an alternative to the more common copper gluconate or copper sulfate. Supplement makers say copper citrate absorbs well and causes fewer digestive problems. Before hauling it home, it’s good to understand what science and real experience say.
Most adults require about 900 micrograms of copper a day, according to the National Institutes of Health. Diets in North America usually meet this recommendation. Supplements often provide 1 to 2 milligrams of copper per day—sometimes far above the daily need.
Too much copper causes harm. Symptoms can include stomach pain, nausea, vomiting, and, over time, damage to the liver and kidneys. Wilson’s Disease, a rare genetic disorder, keeps copper from leaving the body, letting it pile up in organs. Vitamin and mineral supplements skip standard dosages for rare conditions. That means healthy people risk getting too much copper from extra supplementation, especially if multi-vitamins and “immune support” formulas stack copper on top of what food already delivers.
Supplements do not face the same tight scrutiny as prescription drugs in the United States and most countries. Products range in quality. Some brands skip third-party testing. Others go the extra mile—displaying certifications for heavy metal content and purity. Discerning shoppers focus on companies with a strong reputation or those that publish their tests.
Anyone with a known copper deficiency, or issues with the body’s ability to absorb nutrients, might reach for copper citrate. My own grandmother dealt with anemia from a complex intestinal surgery. Her doctor ran bloodwork and guided any use of copper. Most people do not face those challenges and rarely benefit from self-prescribed copper. Food supplies plenty.
Getting copper from supplements instead of meals invites problems. The body absorbs more copper from pills than plants or animals, making overdose easier. People with healthy livers handle this better, but the margin of error shrinks when taking high doses daily. A few studies show certain groups—smokers, pregnant women, and people with specific illnesses—need professional advice before taking extra copper in any form.
Always ask a healthcare provider before starting minerals, including copper. Blood tests confirm true deficiencies and help avoid unnecessary supplementation. For most, a good diet delivers. The food-first approach works: shellfish, seeds, legumes, potatoes, and whole grains all offer copper in natural balance with other nutrients. People curious about supplements should stick to products checked by outside labs and start with low doses.
The bottom line: copper citrate offers an easy-to-absorb form, but daily use should never happen blindly. Facts, tests, and an honest look at diet mark the safest path.
| Names | |
| Preferred IUPAC name | Copper(2+) 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Copper(II) citrate Cupric citrate Copper(2+) citrate |
| Pronunciation | /ˈkɒp.ər ˈsɪ.treɪt/ |
| Identifiers | |
| CAS Number | 866-82-0 |
| Beilstein Reference | 3583723 |
| ChEBI | CHEBI:75960 |
| ChEMBL | CHEMBL3316104 |
| ChemSpider | 20741333 |
| DrugBank | DB14487 |
| ECHA InfoCard | 03c89d9e-6a75-4674-bae4-737d8e62f9e6 |
| EC Number | 208-934-3 |
| Gmelin Reference | 126816 |
| KEGG | C02935 |
| MeSH | D017957 |
| PubChem CID | 24853113 |
| RTECS number | GL7875000 |
| UNII | E52E9G27Y7 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | CompTox Dashboard (EPA) of product 'Copper Citrate': **DTXSID7065448** |
| Properties | |
| Chemical formula | Cu₃(C₆H₅O₇)₂ |
| Molar mass | 399.12 g/mol |
| Appearance | Pale green powder |
| Odor | Odorless |
| Density | 2.6 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -1.7 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa ≈ 3.13, 4.76 |
| Basicity (pKb) | 8.5 |
| Magnetic susceptibility (χ) | -2.1 × 10^-6 |
| Refractive index (nD) | 1.63 |
| Dipole moment | 1.47 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 106 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | A12CX Copper products |
| Hazards | |
| Main hazards | May cause respiratory irritation. May cause eye, skin, and respiratory tract irritation. Harmful if swallowed. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 550 mg/kg |
| LD50 (median dose) | LD50 (median dose): 282 mg/kg (oral, rat) |
| NIOSH | RN0633 |
| PEL (Permissible) | 1 mg/m³ |
| REL (Recommended) | 1.2 mg |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Copper(II) sulfate Copper(II) chloride Copper(II) acetate Copper(II) nitrate Copper gluconate Copper carbonate |