Diammonium hydrogen citrate didn’t pop up in labs by accident. Its roots stretch back to chemists tinkering with citric acid and ammonia salts. Before specialized food additives filled shelves, folks relied on basic chemical understanding. Chemists discovered that mixing ammonium solutions with citric acid offered a reliable source for citrate ions, especially as industries embraced processed foods, cleaning agents, and agricultural inputs. As scientists dug deeper into citric acid salts, diammonium hydrogen citrate found spots in textbooks and research papers. Its growth followed the boom of the chemical industry in the twentieth century—starting as a laboratory curiosity and settling into a staple across labs and factories.
Diammonium hydrogen citrate stands as a colorless, sometimes faintly white, crystalline powder. It comes with a mild acidic taste—thanks to its citric origins—yet brings an alkaline element to the table through its ammonium groups. Suppliers offer it in bulk, with tight packaging to keep moisture out and ensure long shelf life. Food industries lean on it for pH regulation, stabilizing formulations, and offering trace nutrients. It pops up in lab reagent catalogs, food additive indexes, and even cleaning formulations. In real terms, anyone touching pharmaceuticals, food preservation, and agricultural blends probably brushes against this chemical more often than they realize.
Diammonium hydrogen citrate shows up as a crystalline solid, often powdery and slightly hygroscopic. It dissolves easily in water, giving clear solutions, a must-have for food tech applications and analytical chemistry. The compound carries the formula C6H14N2O7, with a molecular weight sitting at about 244.19 g/mol. The melting point sits below the decomposition temperature, hovering around 180°C, where ammonia and citric acid decomposition products start to appear. Thanks to dual ammonium ions, it shows moderate basicity, shifting pH higher than ordinary citric acid solutions. You pick up slight ammonia odor if you do a sniff test close to the pure product, though storage standards keep it sealed away from air and humidity.
Quality control keeps diammonium hydrogen citrate within strict limits. Purity runs usually sit above 98%, with specs drawn to ensure low content of heavy metals like lead or arsenic—down to parts-per-million. Moisture content gets capped below 2%, critical for safe storage and consistent chemical behavior. Labels must show the batch number, content weight, purity percentage, and hazard precautions. Safety info also covers recommended handling instructions and basic toxicology so no one approaches it blindly. Current industry standards call for compliance with REACH regulations and—when used in food—guidance from Codex Alimentarius and local FDA rules. Scan through a technical data sheet, and you'll find pH range, solubility, and parameters like ash content, which flag chemical cleanliness.
The most direct method for making diammonium hydrogen citrate remains the neutralization of citric acid solution with ammonia, usually using ammonium hydroxide. Citric acid—often sourced from fermenting simple sugars with Aspergillus niger—gets dissolved in deionized water. With careful stirring, ammonia solution drips in, pH monitored by electrodes to avoid overshooting into a tri-ammonium species. Heat may be applied for rapid reaction, then cooling leads to slow evaporation or crystallization. Crystals get filtered, washed to knock off excess reactants, then dried in vacuum or under mild heat. This process delivers bulk product with low residual contaminants, depending on water and reagent purity. Some producers recycle process water or recover ammonia, reflecting tighter environmental standards.
Diammonium hydrogen citrate opens the door to a handful of useful chemical moves. As a mild base and a chelate, it binds metal ions—handy for analytical chemists needing to buffer solutions or stabilize minerals in food applications. When heated, it gives off ammonia, pushing the pH downward and sometimes freeing citric acid, a trick used in slow-release fertilizer technology. React it with calcium chloride or magnesium sulfate in water, and you'll see citrate salts form out, switching up properties for specialized industry needs. In organic synthesis, the mild acidity and complexing ability let chemists control rates of reaction and precipitation. Some folks even tailor the chemical by partial neutralization, steering pH for unique food or lab uses.
Diammonium hydrogen citrate appears under several names in catalogs. Watch for ammonium citrate dibasic, diammonium citrate, or even diammonium monocitrate. Old-school literature sometimes lists it as ‘citric acid diammonium salt’. Research labs and suppliers prefer specifications using the CAS registry—CAS 3012-65-5. When reviewing safety paperwork, names might shift depending on country, but the substance itself stays the same. These alternate labels connect everything from food safety codes to chemical procurement databases, ensuring traceability across the supply chain.
Long-term safety sits in the details with this chemical. Direct skin or eye contact could cause mild irritation, so industry workers use gloves and goggles. Dust can irritate respiratory linings if inhaled during handling. In food manufacturing, operators respect regulatory limits for ammonium residues, with regular batch testing. Cleanup follows practices used for mild acids or bases—plenty of water, no strong oxidizers nearby. Storage demands sealed bags or drum containers in cool, dry areas, far from incompatible materials like strong acids. Facilities inspect labels for hazard pictograms per GHS standards, covering accidental exposure or spillage.
Food systems appreciate diammonium hydrogen citrate for its ability to regulate pH and support shelf life. Chefs and industrial processors use it in soft drinks, powders, and preserves where a boost in acidity or buffering capacity keeps flavors consistent. Agriculture takes advantage by using it as a slow-release fertilizer component or nutrient stabilizer, especially when soil management calls for nuanced pH shifts. Lab chemists value it for calibration of pH meters, buffer preparation, and as a mild complexing agent during analyses involving calcium and magnesium ions. In water treatment, the salt keeps scaling down and adjusts mineral solubility. Cleaners and detergents pull from the same bucket, relying on citrate’s mild chelating ability to break up difficult mineral deposits. Pharmaceuticals sneak it in as a pH adjuster and stabilizer, where drug solubility matters.
Universities and corporate labs invest time exploring how diammonium hydrogen citrate interacts in complex matrices—from food to environmental samples. Analytical chemists experiment with detection methods for ammonium and citrate ions, aiming for improved monitoring in food and environmental quality control. Product planners look into microencapsulation, hoping to develop smarter slow-release fertilizers or flavor-blending agents. Formulations expand into biotechnology, where the molecule’s gentle chemistry protects sensitive enzymes and proteins during manufacturing and storage. Environmental researchers test how the compound breaks down under natural conditions, eyeing possible impacts on soil and water quality. Studies continue on blending citrates with other organic acids to probe synergistic effects in cleaning, preservation, and crop science.
Diammonium hydrogen citrate has a safety record supporting its widespread use, but research keeps safety profiles updated. Acute exposure at typical use levels rarely triggers concerns among healthy adults, but concentrated dust in industrial settings can irritate lungs or skin. Chronic ingestion studies in animals point to low toxicity, with rapid metabolism of ammonia and citrates in the body. Large doses could disturb electrolyte balance or aggravate kidney issues, keeping attention focused on dosage in food and therapy products. Recent work looks at long-term soil and water interaction—how leaching could affect freshwater biology at higher application rates in agriculture. Regulatory bodies set conservative daily intake limits, often below actual use, to lock in safety margins as new data emerges.
Looking ahead, diammonium hydrogen citrate stands to gain ground in green chemistry programs, thanks to citric acid’s renewable production and ammonia’s increasing availability from low-carbon processes. Food tech and agriculture anticipate new roles for the compound underpinning next-generation functional foods, shelf-life extenders, and soil enhancers. Synthetic biology lines up fermentation routes to boost purity and bring costs down, letting smaller producers enter markets dominated by large chemical firms. Environmental engineers focus on using citrates in sustainable water treatment, hoping to replace less eco-friendly chelators in large-scale use. For toxicology and safety research, better testing methods promise tighter limits for vulnerable populations and sensitive ecosystems. This compound—rooted in old-school chemistry yet open to modern innovation—will keep drawing interest from anyone who values food safety, productive agriculture, and low-impact, circular manufacturing.
Diammonium hydrogen citrate doesn’t turn a lot of heads by name, but it shows up across industries in ways most folks never think about. Food, farming, and even the lab all borrow a bit of help from this compound. This isn’t some trend you read about in lifestyle magazines. It’s a common ingredient that manages to be both useful and pretty unnoticeable. The thing is, most people have probably used a product involving diammonium hydrogen citrate without ever knowing it.
The most noticeable spot for diammonium hydrogen citrate in daily life comes in food. Take a walk down the grocery aisle. Pick up some powdered drinks or candies—there’s a decent chance you’ll spot it on the label if you look close enough. This compound acts as an acidity regulator. That matters because acidity changes how foods taste and how safe they are to eat. Without it, some drinks would veer too sour or sit too flat. From lemonade powders to gelatin desserts, keeping that sweet spot matters. I remember trying to make lemonade once without balancing out the acid; nobody thanked me for the mouth-puckering outcome. Food makers have learned a lot more trust in measured, food-safe ingredients like diammonium hydrogen citrate than in guesswork.
Farmers see a different side of diammonium hydrogen citrate. Sometimes, soils need a nudge. This compound sometimes lands in the soil as a fertilizer additive. Plants grab nutrients more easily when soil conditions deliver the right balance, and helping make minerals more accessible can mean better crops and fewer problems for farmers. It’s not some miracle solution, but it sits on the shelf among the mix of fertilizers chosen for getting crops to market with less trouble.
Outside the farm and kitchen, laboratories lean on this chemical too. Chemists use it for preparing buffer solutions, making sure experiments run with the right pH. In my college days, making these buffers was a regular job. If pH wandered, the whole experiment could fall apart—sometimes hours of work lost over a careless mix. Diammonium hydrogen citrate gives precise, reliable control, helping students and professional researchers alike get results that make sense. Small details like this keep science on track.
Using any food additive or agricultural input safely should never be taken for granted. Diammonium hydrogen citrate has cleared regulatory review in many countries. That means consumer watchdogs and food safety agencies have reviewed science about risks and limits. Anyone worried about new ingredients has a right to know what’s in their food. Transparent labeling helps. Reading packages and understanding what gets added pays off over time, especially with allergies and health goals on the line.
In the food and agriculture world, safety and transparency steer the conversation. Companies benefit by sharing testing data and making sure production matches international standards. And there’s always room for more studies on long-term health effects. As new research gets published, scientists and regulators may update guidelines. For now, diammonium hydrogen citrate has earned its role across kitchens, fields, and labs, proving that a small ingredient can quietly support plenty of daily routines.
Diammonium hydrogen citrate sounds like something reserved for a chemistry lab, but it shows up in things like soft drinks, flavored waters, and even some candies. Most folks have tasted it, probably without realizing it, since it gets added to boost tartness or to help balance out formulas in packaged foods. Its job resembles what citric acid does: keep drinks bright, preserve shelf life, and support the right pH in products.
I sometimes look at ingredient lists and wonder how to pronounce half the things in there, let alone know if they are safe. Diammonium hydrogen citrate comes from citric acid, which we find in lemons and limes. Food manufacturers combine citric acid with ammonia to get it. This ingredient does get the green light from food safety regulators like the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA). Regulatory agencies say it belongs on the Generally Recognized as Safe (GRAS) list when used in the small amounts that show up in food.
Studies have looked at this compound for toxicity or weird side effects. At the doses people typically eat, it hasn’t popped up as a problem. The ammonia part sounds worrisome, but our bodies know how to process small amounts. As someone who reads up on nutrition science, I pay close attention to real-world evidence, not just test tubes or animal labs. Decades of use haven’t raised red flags in healthy people.
Now, too much of anything can steer us into risky territory. In food science, the magic lies in the dose. Eat handfuls of pure citric acid or anything similar and it’ll upset your stomach. Diammonium hydrogen citrate fits that pattern: safe as an additive, but not meant for straight consumption on its own. The occasional soft drink won’t tip the balance.
Some people have to be extra careful, especially anyone with kidney disease. Citrates can shift the body’s acid-balance, which the kidneys control. If you have known trouble handling acids or managing kidney stones, it makes sense to talk to a doctor, especially if you’re drinking supplements or using products that contain extra citrate.
I’ve noticed friends with food allergies always reading fine print. Diammonium hydrogen citrate doesn’t trigger allergic reactions the same way as nuts or dairy. For most, it flies under the radar. But folks with very specific metabolic conditions might want to look at labels and ask professionals if they notice recurring symptoms.
Big food companies should always keep consumers informed. I trust brands that include clear labels and plain explanations more than ones full of jargon. More public information about additives like this would help people make better choices. No one likes surprises, especially in the things we feed our families.
Easy-to-read ingredient lists could empower folks to make their own decisions. Clarity creates trust and lets everyone feel more comfortable about what shows up at the dinner table.
Diammonium hydrogen citrate sounds like a mouthful, but it boils down to a blend of familiar parts. At its core, you find citric acid, that tangy compound even the most reluctant science student recognizes from lemons and limes. Adding two ammonium ions gives this molecule a special twist, along with a single hydrogen joined to the citrate backbone. Put this all together and chemists write its formula as (NH4)2C6H6O7. If you’ve spent time on the ingredient side of things, this formula carries meaning—clear ratios and defined atoms that explain why it behaves the way it does.
Chemicals get a bad rap at times, but diammonium hydrogen citrate plays a helpful role in more than one industry. For those curious about where formulas like (NH4)2C6H6O7 show up, take a walk down the aisles of a food processing plant or a lab supply shop. Its use as a buffering agent and acidity regulator matters. In my time shadowing a food technologist, I found that precise pH control can make or break a recipe. Small tweaks in formulas can affect texture, safety, and shelf life for foods ranging from cheeses to beverages. Citrate salts keep things predictable, especially in large-scale production.
Research supports this widespread use. The Food and Drug Administration includes similar salts on its generally recognized as safe (GRAS) list. These compounds break down naturally and haven’t shown chronic toxicity in evidence reviewed so far. As with anything, safe use means solid training and sound procedures. Cooks and chemists treat every additive with respect, leaning on regulatory documents and safety data sheets to guide them.
Some may ask why formulas and chemistry lessons claim any real-world significance. It’s about trust and transparency. Food safety depends on people understanding what they put in their bodies. At home, most folks want products labeled clearly and honestly. In an age when synthetic additives spark concern, explaining the backbone of compounds like diammonium hydrogen citrate helps everyone get on the same page. If you know a food contains a salt derived from citric acid and ammonia, and you understand their roles, you’re less likely to fall for misinformation.
I’ve watched parents check ingredient lists, hunting for names that sound dangerous. A quick search shows all sorts of online myths, but understanding what a formula stands for gives you tools to push back against panic. Science and food safety go hand in hand; formulas like (NH4)2C6H6O7 unlock context and clarity.
Education matters every step of the way. Schools that teach real-world chemistry often end up growing curious, confident consumers. Short videos, public talks, and hobbyist experiments using kitchen chemistry help demystify technical jargon. When parents involve kids in cooking and explain why certain ingredients do what they do, the gap between scientific names and food on the plate shrinks. Authorities, too, should keep updating food safety guidelines with clear explanations and resources so everyone can access information that is accurate, not just alarming.
If the goal is safer, smarter eating and working, simple clarity wins. Understanding diammonium hydrogen citrate—down to its formula—offers a tangible step in that direction.
Diammonium hydrogen citrate shows up in labs, food production, and even industrial settings. I’ve seen mistakes happen just from simple oversights, and it doesn’t matter if someone’s dealing with a few grams for an experiment or a full pallet in a warehouse—storage matters. Without some care, chemicals like this tend to lose their punch, or worse, cause trouble.
A clean, dry storage area keeps diammonium hydrogen citrate in shape. Any contact with moisture slowly breaks down these types of salts, affecting both shelf life and performance. I still remember opening a container that had been kept in a corner with some condensation problems—the contents caked up, and we had to toss the lot. It only takes a little humidity to start that clumping process.
So it makes sense to always use airtight containers—preferably original packaging, and if that’s opened, moving the remainder to a well-sealed jar or drum. Keeping it off the floor, away from pipes and walls, also helps avoid problems caused by condensation or accidental spray from a leaky foundation.
Some warehouses keep rooms warm or use no climate control at all. Higher temperatures speed up degradation or cause chemical changes that throw off careful formulas. On the other hand, storing diammonium hydrogen citrate in cold, wet spaces can result in water absorption or cause the substance to harden and become unusable. Based on manufacturer guidance and experience, room temperature is usually best. That means aiming for somewhere in the range of 15-25°C (59-77°F). Consistency works better than chasing a perfect number that'll shift with each season.
Direct sunlight also takes a toll. Light can trigger breakdown in chemicals, or even heat up plastic containers until they warp. Shelving in shaded rooms or cabinets does a better job than sticking chemicals under skylights or right by windows.
Diammonium hydrogen citrate doesn’t spark fear the way strong acids or volatile compounds do, but a label doesn’t tell the whole story. Handling powders always brings the risk of accidental inhalation. In my own routines, I’ve put on a simple dust mask before opening any bag, even if just for a quick measurement. That quick step keeps accidental exposure to a minimum.
Every container storing this ingredient should carry a clear, intact label. That means batch number, date received, and hazard information right up front. Confusion in the storeroom can lead to expensive mistakes or riskier errors. Replacing faded stickers or marking up relabeled jars takes a minute but saves a bunch of headaches later.
Regular inventory checks can prevent surprises. Rotating stock by moving older containers to the front or top shelf gets them used before they break down. In larger spaces or sites with multiple chemicals, simple logbooks turn out to be the best network—a quick glance tells everyone what arrived last and which lots should be finished first.
In any work area, clear signage about safety and storage conditions helps remind everyone of the standards. It helps newcomers, and it keeps old habits sharp. Keeping things organized, dry, and labeled costs less than replacing spoiled chemical stock—or solving a preventable safety incident. Storage isn’t fancy, but it’s where real expertise makes life simpler and safer for everybody.
Diammonium hydrogen citrate isn’t some rare compound sitting unused on a laboratory shelf. It pops up more than you might think, especially if you like clear sodas or know about food preservatives. The burning question: does it dissolve in water? Short answer—absolutely, and faster than sugar cubes in hot tea. The real importance of this fact goes deeper. Solubility shapes how safely and effectively the chemical gets used in the products people enjoy or rely on every single day.
Ask anyone who’s mixed kitchen ingredients: if something won’t dissolve, good luck getting a consistent taste or effect. The same principle goes for Diammonium hydrogen citrate. In water, it breaks apart into ammonium and citrate ions effortlessly. Manufacturers depend on that property—whether they’re blending sports drinks or making nutritional supplements smoother and less gritty. Water carries those ions everywhere in the solution, ensuring every sip or slice offers the same results.
Chemists point to a number called “solubility” and back it up with numbers from databases like the Merck Index and PubChem. Diammonium hydrogen citrate clocks in at over 200 grams per liter at room temperature. Even cold tap water takes it in with no trouble. This matters for anyone worried about residue, waste, or even cost. Nobody likes wasting raw ingredients or battling unappealing clumps at the bottom of a tank.
Why care about something so specific? Food safety rides on more than just what goes into a product. How thoroughly ingredients mix changes the risk of uneven flavor, spoilage, and even allergic reactions for some folks. Water-soluble ingredients, including Diammonium hydrogen citrate, help manufacturers avoid these pitfalls and keep production lines humming.
The clear solubility also means fewer surprises for people using it at home or at work. Mix it with water and expect a crystal-clear solution—none of that sandy grit left behind. For bakers, brewers, or home chemists, that reliability saves both time and frustration. It also means less stress during cleanup. Every bit dissolves, and trace residues rinse away without a second thought.
Some folks see “ammonium” in a chemical name and start worrying—fair enough. But Diammonium hydrogen citrate doesn’t build up or linger in water supplies. Once dissolved, its breakdown products pose minimal harm. The Environmental Protection Agency points to rapid biodegradation and low toxicity as reasons it shows up on the “generally recognized as safe” (GRAS) list. Still, it matters that responsible use takes center stage: no dumping into rivers, no overuse in fields or factories.
Despite its easy solubility, mistakes sometimes creep in. Overdosing, sloppy mixing, or ignoring temperature can turn a perfectly clear solution into a cloudy mess. Companies can fix those slip-ups by sharing better recipes, training workers, and using more precise equipment.
Open data from scientific journals and public safety agencies fuels smarter choices. As more information lands in the hands of teachers, food producers, and ordinary consumers, risky practices tend to shrink. More transparent labeling would help as well—so people know exactly what’s in their drinks or preserves, and why each ingredient behaves as it does.
The fact that Diammonium hydrogen citrate dissolves so well in water matters most to those working for safe, reliable, and cost-effective food production. Backed by public science and practical experience, this trait helps keep things consistent on both small and industrial scales. Keeping an eye on training, communication, and environmental guidelines closes the circle, turning a simple chemical property into an everyday advantage.
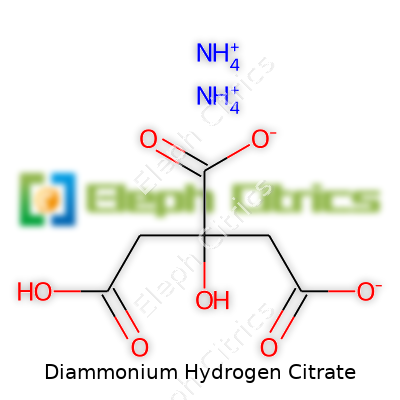

| Names | |
| Preferred IUPAC name | diammonium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Diammonium citrate Ammonium citrate dibasic |
| Pronunciation | /daɪ.əˈmɔːni.əm ˈhaɪdrə.dʒən ˈsɪtrət/ |
| Identifiers | |
| CAS Number | [Diammonium Hydrogen Citrate CAS Number: "3012-65-5"] |
| Beilstein Reference | 3597084 |
| ChEBI | CHEBI:63038 |
| ChEMBL | CHEMBL1201567 |
| ChemSpider | 41660 |
| DrugBank | DB13026 |
| ECHA InfoCard | ECHA InfoCard: 03-2119981506-28-0000 |
| EC Number | 205-527-1 |
| Gmelin Reference | 145715 |
| KEGG | C14647 |
| MeSH | D007642 |
| PubChem CID | 162112 |
| RTECS number | GE7250000 |
| UNII | R92DOX505O |
| UN number | UN3077 |
| Properties | |
| Chemical formula | (NH4)2HC6H5O7 |
| Molar mass | 210.18 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.4 g/cm³ |
| Solubility in water | soluble |
| log P | -3.14 |
| Acidity (pKa) | 3.1 |
| Basicity (pKb) | 1.5 |
| Dipole moment | 3.09 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 389.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -207.6 kJ/mol |
| Pharmacology | |
| ATC code | A12AA22 |
| Hazards | |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P264: Wash hands thoroughly after handling. |
| Lethal dose or concentration | LD50 oral rat 2820 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2820 mg/kg |
| NIOSH | SN4260000 |
| PEL (Permissible) | Not established |
| Related compounds | |
| Related compounds |
Ammonium citrate Trisodium citrate Monosodium citrate Citric acid Monopotassium citrate |