Back in the early chapters of organic chemistry, malic acid drew plenty of interest for its role in the flavor of fruits and its place in metabolic cycles. Chemists looking to tweak its properties started experimenting with various esters, and that’s how dimethyl malate entered the scene. As labs pushed for better solvents, intermediates, and specialty chemicals, the double methylation of malic acid essentially created a new opportunity. By the late 20th century, dimethyl malate wasn’t a novelty; it had become a staple for synthesis and analysis in academic settings and factories alike. This background shapes the way people look at it today—less as some exotic compound, and more as a practical solution born out of necessity and curiosity.
Dimethyl malate comes across as a straightforward molecule. Its core—malic acid—brings its own legacy, contributing backbone to many chemical reactions. Swap the two carboxyl groups’ hydrogen atoms for methyl groups, and the resulting ester offers properties that plain malic acid can’t match. Chemists value it for this change. The molecule offers more stability under certain lab conditions. It moves quickly through reactions where the acid form slows things down. Instead of worrying about decomposition or aggressive reactivity, handlers find a friendlier, more predictable companion in dimethyl malate.
In the bottle, dimethyl malate usually appears as a clear, colorless liquid. It brings a faint, fruity odor—not entirely surprising given its parentage. Its melting point sits well below room temperature, which means it doesn’t solidify without intentional cooling. The boiling point typically hovers just above 200°C, providing reasonable thermal stability. Dimethyl malate dissolves in many organic solvents due to those methyl groups, but it resists mixing with water. Chemically, it behaves as you’d expect from a diester of a dicarboxylic acid: mild reactivity, with potential for transesterification and participation in various nucleophilic substitutions.
Factories and research suppliers selling dimethyl malate usually guarantee purity levels above 98%. The chemical formula runs as C6H10O4. Labels might read “Dimethyl (2R)-2-hydroxybutanedioate” or use other synonyms. Typical labeling includes hazard symbols indicating skin and eye irritant, along with proper storage advice: keep out of sunlight, store below 25°C, and use only in well-ventilated areas. Packaging varies between amber glass bottles for lab use and food-grade drums for bulk requests, always sealed tightly against moisture and contamination. Safety data sheets explain procedures for accidental spill or exposure, emphasizing immediate washing of skin and medical attention for inhalation or ingestion.
Most commercial dimethyl malate comes from direct esterification of malic acid with methanol, using concentrated sulfuric acid or another dehydrating agent as catalyst. Heating the mixture drives off water and pushes the equilibrium toward ester formation. Modern setups recycle unreacted methanol to cut waste and improve yields. Once the reaction ends, the solution cools, and the crude product gets washed, neutralized, and purified by fractional distillation or sometimes extraction. Experienced operators focus on gentle temperatures and tight pH control to prevent breakdown—results speak for themselves through high purity and consistent product.
Dimethyl malate turns up often as an intermediate in organic synthesis routes. The ester groups yield to hydrolysis when someone wants to regenerate malic acid. With the right nucleophiles, it serves as a jumping point for preparing substituted malates. Chemists also use it in the Diels-Alder and Michael addition reactions where the ester groups draw in reactive partners. Hydrogenation can remove the double bonds, while careful oxidation targets the secondary alcohol. Each reaction stems from the well-placed functional groups that keep the molecule reactive just enough, but not reckless.
No one speaks a single language when it comes to chemicals. Dimethyl malate goes by several names across suppliers, papers, and borders. Commonly, it appears as “Dimethyl malate,” “Malic acid dimethyl ester,” and in IUPAC form as “Dimethyl 2-hydroxybutanedioate.” The (R) or (S) prefix sometimes marks the chirality. Retailers in certain regions throw in trade names or catalog numbers, but the structure always traces back to the same backbone. This confusion slows down procurement for anyone not cross-checking every alias—accuracy in purchasing starts with knowing these name games.
Handling dimethyl malate calls for routine lab precautions—Nitrile gloves, splash goggles, and fume hoods stay in use. Exposure risks line up mainly around mild irritation to eyes, skin, and the upper respiratory tract. I’ve breathed vapor from spilled ester in a poorly ventilated extraction room before, and the quick burn in sinuses showed just how important good air flow becomes. Storage containers need secure, leakproof seals, and leftovers should never get poured down the drain. Fire hazard remains low compared to more volatile esters, but the material safety data sheet always sits close in busy labs. Long-term studies show no chronic toxicity at low concentrations, but accidental ingestion or sustained skin contact can bring acute problems ranging from rash to headache.
Research, manufacturing, and even food science tap into dimethyl malate’s strengths. Chemists use it as an intermediate in synthesizing new pharmaceuticals—the ester groups mask carboxylic acids during multi-step reactions, only to reveal those groups later with basic hydrolysis. Some labs pick dimethyl malate as a chromatographic reference. Polymer producers appreciate its compatibility as a plasticizer or monomer building block. Food technologists have explored it for flavor modification and aroma enhancement, since its reactivity and volatility can subtly boost fruit flavors. Despite this reach, most demand comes from specialty chemical production rather than consumer goods.
Current research swirls around better derivatization of dimethyl malate to produce performance materials. Teams at several universities delve into new biodegradable polyesters made by copolymerizing dimethyl malate with lactones or other bio-sourced monomers. Medicinal chemists design new prodrugs using dimethyl malate’s esters to mask bitterness and enhance absorption in the gut. Sustainable chemistry pushes for greener synthesis—catalysts borrowed from enzyme-mimics trim energy needs and waste output. My own work with undergraduate teams tested alternative dehydrating agents for esterification, aiming to cut environmental cost without sacrificing product yield.
Toxicologists agree that dimethyl malate does not rank among the most dangerous chemicals under standard lab and industrial use. Acute exposures lead to mild symptoms, typically limited to skin and sensitive membranes. Studies in rodents suggest low oral toxicity, with high oral doses causing minor gastrointestinal upset. In vitro experiments show no mutagenic activity, but thorough chronic testing remains thin. Environmental impact gets mitigated by rapid hydrolysis and breakdown, provided disposal keeps to controlled environments. Regulators still urge caution—unknowns and edge cases always complicate new uses. Practical experience in both academic and commercial settings points to careful, informed use as the best defense.
With industries shifting toward greener, safer chemicals, dimethyl malate has room to grow. Its bio-based origin in malic acid and straightforward modification suit it for future specialty polymers and sustainable materials. If catalysts keep improving and purification gets easier, prices could drop and open doors for wider commercial uses. Medical researchers see promise in further developing prodrugs and taste-masking agents. Regulatory landscapes call for deeper data on chronic exposure and breakdown pathways. As synthetic biology scales up, engineered microbes could generate malic acid derivatives from renewable feedstocks. The next decade will likely see more patents and academic papers, with dimethyl malate securing new roles far beyond its current borders.
Dimethyl malate shows up in places a lot of people wouldn’t expect. As an organic compound made by esterifying malic acid, it differs from familiar industrial chemicals in subtle ways that matter. My own experience in chemical analysis made me appreciate how small tweaks in a molecule can open new doors for application. Dimethyl malate finds real value in both research and industry because of its special structure and reactivity.
Labs and manufacturers use dimethyl malate as a building block. Researchers depend on it for clean, manageable reactions when making new chemicals. Its two methoxy groups allow for selective chemical changes without unpredictable byproducts. Pharmaceutical chemists rely on purity and predictability, and dimethyl malate delivers on both. Reliable starting materials set the stage for smarter drug development and material science, fields where timing and reliable outcome keep costs in check.
Food scientists spend a lot of time trying to capture and recreate natural flavors. The malic acid backbone in dimethyl malate plays a part in mimicking the tartness you get from apples and other fruits. Flavors don’t just enhance taste—they drive consumer choices and influence everything from soft drinks to candies. Dimethyl malate gives product developers a precise way to introduce sour notes without introducing instability or unwanted flavors that sometimes tag along with other acids.
Specialty plastics don’t always make headlines, but they shape our day-to-day lives in quiet ways. Polymer chemists use dimethyl malate to adjust flexibility in plastics and resins. By changing the hands holding the polymer chains, they control how stiff or bendy a material ends up. In my time working with specialty coatings, I saw how the right chemical tweak can keep cars shiny, furniture scratch-resistant, and electronics safe from heat. Small components like dimethyl malate often play an outsized role.
Every useful chemical brings some baggage. Dimethyl malate isn’t toxic like some industrial solvents, though that doesn’t mean anyone should handle it carelessly. Safety goggles, gloves, and solid ventilation matter—a lesson I’ve learned firsthand running late-night syntheses in busy labs. We lean on safety data and transparent sourcing so that heightened demand doesn’t cut corners on safe handling.
Easy access to pure dimethyl malate supports innovation at every scale. Open supply chains and clear labeling build trust, especially for brands watching public perception and regulatory changes. Chemists need to know where their materials come from and whether suppliers cut corners with impurities. Gaps in traceability carry real risks, so tightening up that system protects workers and end users while keeping projects moving.
People working in R&D, flavor development, or advanced materials will keep counting on dimethyl malate. The search for eco-friendly chemicals pushes everyone to examine the full picture—from raw material sourcing to responsible disposal. Dimethyl malate shows how a small molecule can link technology, food, and safety. It stands as a reminder that chemistry shapes our world both in the lab and on the shelf, quietly supporting advances that matter every day.
Scientists and manufacturers encounter all sorts of molecules, but only a handful offer the versatility of dimethyl malate. This compound carries the chemical formula C6H10O5. In the lab, it’s created by taking malic acid and reacting it with methanol, forming the dimethyl ester. Anyone who’s handled esters knows they often bring distinct characteristics, whether for chemical synthesis or applications in flavors and fragrances.
Dimethyl malate’s formula isn’t just a string of numbers and letters from a textbook; it provides crucial information for chemists. Six carbons, ten hydrogens, and five oxygens—this arrangement helps researchers predict how the molecule will behave during a reaction. If you’ve ever followed a synthetic route or measured out reagents, knowing these little details can save endless headaches down the line. Getting one atom wrong tends to throw off purity, yield, and performance in nearly every context. For a researcher, that spells lost time and wasted resources.
I’ve seen first-hand how the structure of a compound like dimethyl malate opens the door to multiple uses. Because it’s a diester, it fits into organic synthesis as an intermediate—offering chemists a reliable starting point for creating more complex compounds. In academic settings, undergrads and grad students alike study these sequences to better understand how molecules fit together and break apart. Beyond the classroom, flavor chemists make use of esters for their scents and tastes. Dimethyl malate sometimes pops up as a building block for these creations, highlighting how simple chemistry underpins much of our daily sensory experience.
Despite the straightforward formula, working with dimethyl malate asks for a level of attention to detail. Forgetting to double-check the purity can lead to side reactions in the lab. Storage conditions make a difference, too. With many esters, exposure to moisture or high temperatures means the risk of decomposition rises. A sealed container, a cool room, and clear labeling go a long way toward keeping reactions consistent and results reproducible. Good lab practice isn’t just about ticking boxes—it’s about protecting your data and your health. Simple safety steps pay off over months of experiments.
Trust in chemical formulas like C6H10O5 rests on transparency and verification. Analytical methods—thin layer chromatography, NMR, or mass spectrometry—aren’t just academic exercises. They build confidence that what’s in the bottle matches what’s on the label. Years ago, I saw an entire series of reactions fail because one batch came with mislabeled content. The lab had to toss results and start over. Taking the time to confirm the structure at the outset always proves worthwhile.
Dimethyl malate doesn’t get headlines, but its formula provides real insight for professionals across multiple fields. Chemistry relies on clear communication, sound data, and practical applications. Keeping track of something as specific as C6H10O5 helps both new and seasoned chemists avoid costly mistakes and keep their work moving forward. With reliable knowledge, everyone from academic researchers to industrial specialists can contribute better results and safer processes to the wider world.
Dimethyl malate pops up in chemical catalogs and research labs every so often, especially among folks working in organic synthesis or flavor chemistry. It flows clear and colorless in a bottle, easy to measure and blend. The thing that always jumps out: every substance on a lab shelf has its quirks, and this one is no exception. Many overlook the basics because it doesn’t sound as threatening as some chemicals with skull and crossbones, but the truth is, there’s more to the safety story than meets the eye.
Dimethyl malate doesn’t rank as a famous hazard, and that can lull researchers or technicians into a false sense of security. A quick scan of its safety data sheet points to some real risks. The compound gives off an odor, hinting at its volatility. Vapors mean inhalation exposure, and if you breathe it in, throat and lung irritation can follow. If any lands on skin or splashes toward your eyes, mild to moderate irritation becomes likely. I’ve watched seasoned lab workers skip gloves or goggles, and the unfortunate result is usually a sprint to the eyewash or sink—sometimes with red skin or watery eyes.
Handling dimethyl malate reminds me of the early years in chemistry, where the lesson hit early: don’t assume “mild” means “harmless.” During synthesis work, pouring or transferring without proper ventilation felt okay until the fumes tickled the nose and throat. Peers sometimes scoff at the prospect of running a fume hood for something as unassuming as a malate ester. Yet over time, the subtle effects stack up—headaches, an irritated nose, drying hands. Each little incident chips away at focus and well-being.
Research does not show persistent, cumulative toxicity with dimethyl malate, and animal studies are rare. Its breakdown products resemble those of malic acid, found naturally in fruits. That offers some reassurance. But most safe handling guidelines come not from medical disasters, but from paying attention to short-term warning signs. Consistent exposure, even at low levels, is best avoided. The science doesn’t rule out possible allergies, respiratory issues, or skin sensitivity developing after repeated mishandling.
Routine matters: keep gloves and goggles handy, keep jars sealed, and run the fume hood—especially in confined spaces. Good ventilation, sturdy PPE, and a culture of safety speak louder than reassurance from chemical property sheets. Supervisors aiming to support healthy working habits take a few simple steps: clear access to the SDS, posting reminders about PPE, and scheduling regular safety briefings. It’s not just about ticking boxes; it’s about building habits that help people work smarter.
From personal experience, the labs that run smoothly are the ones where people respect every chemical, no matter how “benign” the label. Shared experience trumps complacency. Newcomers learn fast from mentors who model proper handling, and the air stays cleaner, the hands stay healthy. No need for paranoia, just respect and know-how. Dimethyl malate serves as a good reminder: common sense, proven procedures, and a dash of humility go a long way toward keeping everyone safe for the long haul.
Dimethyl malate can show up in a couple of industries, from pharmaceuticals to chemical manufacturing. Chemists and buyers both put a lot of weight behind the question: what’s in the bottle, and how pure is it? This matters because impurities in a batch don’t just mess with quality—they sometimes threaten safety or lead to wasted resources. Pure chemicals keep a process flowing right, whether you’re making new drugs or tweaking materials for food additives.
The standard purity for dimethyl malate rolls in at 98% or better, according to big global suppliers. Lab-grade batches might read even higher. A batch at 97% versus 99% purity does more than set a number on a label. That 2% left over could mean extra solvents, leftover reagents, or moisture. In industrial settings I've seen, those trace materials sometimes spell disaster—nobody wants to run a catalyst reaction that fails because of a stray impurity.
Testing always comes before claiming a purity number. Chemistry teams break out tools like gas chromatography, high-performance liquid chromatography (HPLC), and infrared spectroscopy. These techniques catch differences as small as 0.01%. A clean peak on an HPLC machine can boost confidence that the sample actually reaches the advertised purity.
Melting point and boiling point checks also come into play. Dimethyl malate, in its pure form, melts and boils in tight temperature ranges. If a batch softens a few degrees off the mark, that's a clue it carries unwanted guests. Companies run moisture analysis because water tends to creep in during packaging and storage. I've run material where moisture content alone pushed a batch out of spec, creating sticky messes or throwing off reactions downstream.
Drilling down to greater than 98% purity helps finished products meet regulatory demands. Drug-makers stake reputations and patient safety on consistency. In one case with pharmaceutical intermediates, a tiny bit of byproduct caused harsh allergic reactions. That example sticks with people who work in quality control. Even outside of pharma, users expect reliability—plasticizers or specialty chemicals rely on pure starting materials so colors stay stable, and properties match data sheets.
Impurities can show up due to poor synthesis steps, dirty glassware, or low-quality starting reagents. Catching those before bad material goes out the door means spending less on callbacks, and avoiding regulatory headaches. As someone who’s seen products recalled for trace solvents, those stories make a strong point for careful testing and reliable documentation in every chemical shipment.
Sourcing always starts with a certificate of analysis. Reliable suppliers stick with ISO or GMP standards, listing out strengths, weaknesses, and lab results for each lot. Third-party labs give another check. Auditing suppliers and running spot checks in-house keep everyone honest.
Storage and handling practices matter, too. Dimethyl malate should stay sealed tight, away from heat and damp air. Trained staff, detailed procedures, and frequent calibration of testing instruments close the loop. Quality doesn't just come from the lab—it builds from a culture where people pay attention and fix issues before they leave the warehouse.
Purity isn’t just a technical stat in the world of chemistry. It carries a story about safety, trust, and hard-earned reputation. Companies do best when they push for thorough testing and transparent reporting, not just to tick off a regulatory box but to ensure every product performs the way it should every single time.
I’ve worked with enough chemicals in labs and storage rooms to know that every compound brings a unique set of challenges. Dimethyl Malate stands out because even though it’s not as infamous as some other esters, mistakes in storage can mean trouble for both people and the product itself. Safety starts with understanding the material, so let’s look at what experience and some practical science say about keeping it stable and safe.
Chemicals like Dimethyl Malate react with the environment. Ask anyone who’s cleaned up a spill: esters can catch fire more readily than most would guess. Keeping this compound away from heat sources and direct sunlight gives you a fighting chance against accidents. Forget about storage in utility closets or next to steam lines. Reliable buildings set up shelves in cool, dry areas where temperature stays below 25 degrees Celsius. That reduces evaporation and limits fire risks. Moisture in the room can also impact the purity, so a desiccator or at least sealed storage is essential. Properly sealed containers cut down the chances for water vapor to sneak in and cause hydrolysis or weird smells. I’ve seen labs lose entire batches because folks left caps loose — it’s an expensive and frustrating lesson.
Experience tells me that glass offers the best defense against chemical breakdown, so skip plastics unless verified as chemically compatible with esters. Polyethylene and polypropylene sometimes react over time, leading to leaks or contamination. Amber glass shields against UV light, which further keeps reactions at bay. Factories often choose heavy-duty bottles with tight seals that don’t crack under slight temperature changes, since tiny cracks can lead to big spills.
A lot of accidents happen because people underestimate small leaks. Floors in storage areas should be easy to clean and resistant to chemicals. Secondary containment, maybe as simple as a deep tray or a fume hood, doubles up protection. This isn’t paranoia. If a broken bottle goes unnoticed, volatile fumes can build up. More than once, I’ve been grateful for a well-placed spill kit nearby. Heavier anhydrous salts for neutralization help in emergencies, and clear signage helps workers know exactly where to go, even under stress.
Even the best setup fails if no one knows what’s inside the container. Plain labeling helps everyone identify the risk. Include chemical name, concentration, and hazard information. Training new employees isn’t just about ticking boxes; it’s about building habits so no one mistakes one clear liquid for another. In my own experience, drills and refreshers matter a lot more than binders stuffed with protocols. Safety data sheets in an accessible spot take some of the guesswork out of handling spills or accidents. Familiarity with the risks means fewer mistakes and a more confident team.
Dimethyl Malate won’t cause problems so long as storage pays respect to its chemistry. With cool temperatures, tight seals, and the right container, losses and risks drop sharply. Accidents decline in a place where safety isn’t just a sign on the wall but a part of everyday action. My years dealing with chemical storage teach me small investments yield big rewards, and shortcuts usually end up costing far more than you save.
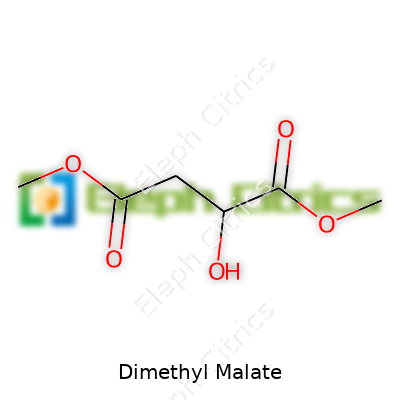
| Names | |
| Preferred IUPAC name | Dimethyl 2-hydroxybutanedioate |
| Other names |
Dimethyl 2-methylpropanedioate Dimethyl propane-1,3-dioate Dimethyl 2-methylmalonate Malonic acid dimethyl ester Dimethyl propanedioate |
| Pronunciation | /daɪˈmiːθəl ˈmæleɪt/ |
| Identifiers | |
| CAS Number | 616-05-7 |
| Beilstein Reference | Beilstein Reference: 1721324 |
| ChEBI | CHEBI:131100 |
| ChEMBL | CHEMBL50424 |
| ChemSpider | 71323 |
| DrugBank | DB14653 |
| ECHA InfoCard | ECHA InfoCard: 100.043.688 |
| EC Number | 2466-03-7 |
| Gmelin Reference | 7138 |
| KEGG | C05379 |
| MeSH | Dicarboxylic Acids |
| PubChem CID | 8731 |
| RTECS number | OH8225000 |
| UNII | 9A8A47H5I0 |
| UN number | UN2528 |
| CompTox Dashboard (EPA) | DTXSID8014365 |
| Properties | |
| Chemical formula | C6H10O4 |
| Molar mass | 160.17 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | Fruity |
| Density | 1.18 g/cm³ |
| Solubility in water | Soluble |
| log P | -0.35 |
| Vapor pressure | 0.02 mmHg (25°C) |
| Acidity (pKa) | 5.62 |
| Basicity (pKb) | 10.64 |
| Magnetic susceptibility (χ) | -44.0e-6 cm³/mol |
| Refractive index (nD) | 1.416 |
| Viscosity | 1.684 mPa·s (25 °C) |
| Dipole moment | 2.99 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 234.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -958.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1542.0 kJ/mol |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P261, P264, P271, P272, P280, P302+P352, P305+P351+P338, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-1-0 |
| Flash point | 76 °C |
| Autoignition temperature | 190 °C |
| Lethal dose or concentration | LD50 (oral, rat): 3200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 1100 mg/kg |
| NIOSH | MMW |
| PEL (Permissible) | Not established |
| REL (Recommended) | 500 mg/kg |
| Related compounds | |
| Related compounds |
Diethyl malate Methyl malonate Dimethyl succinate Dimethyl fumarate |