Chemists isolated citric acid centuries ago, but scaling up its production proved challenging for a long time. In the early 20th century, as the food and pharmaceutical industries boomed, sodium citrate came into focus for its ability to improve flavors and control acidity. Disodium citrate, with two sodium atoms per citrate molecule, moved quickly from lab benches to manufacturing floors. Researchers in the 1930s noticed how it buffered acidity more gently than plain citric acid or trisodium citrate, making it a handy ingredient in both foods and medicines. Factories in Europe and North America ramped up production, taking advantage of cheap raw materials and the growing need for additives that stabilized products and prolonged shelf life. Over time, its role expanded far beyond food, popping up in chemistry labs, cleaning agents, and even as a medical additive.
Disodium citrate, known among chemists for its reliability, appears as a colorless or white crystalline powder. It dissolves nicely in water but not in alcohol. Food technologists choose it for its stable sourness and gentler impact on taste buds compared to stronger acids. Pharmaceutical companies rely on it to control drug acidity in liquid medicines. The compound shows up under names like E331(ii) and sodium hydrogen citrate, but inside any pack or drum, you find the same tart, salty powder developed through careful process controls. It stands out for its predictability and versatility in a world full of far more finicky ingredients.
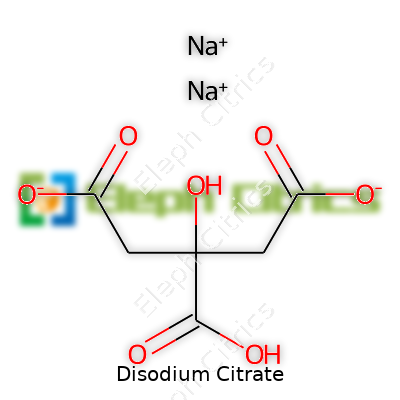
Disodium citrate’s formula is Na2C6H6O7. The powder gives off no smell and ranges in appearance from fine to grainy. It does not clump easily when kept dry, which may sound simple but means a lot if you stockpile warehouses. Melting occurs at temperatures just above 200°C, where it starts to break down and lose water. Water absorbs the compound easily, and with a solubility that exceeds 600 grams per liter at room temperature, it outperforms many similar salts. Good shelf stability means the compound holds up without breaking apart, even in humid or warm climates, as long as storage stays dry. The moderate alkalinity—pH around 7.6 to 8.6 in a 5% solution—sets it apart from citric acid, giving more freedom in recipes that demand stable pH.
The ingredient appears most often under the E number E331(ii) and “Disodium Hydrogen Citrate.” Food-grade product requires more than 99% purity, low moisture content, and absence of heavy metals to meet tough regulations. Standard labeling lists chemical name, purity percentage, batch code, production date, and manufacturer. Pharmaceutical and food authorities enforce these details. Labels on export batches often include both CAS number (144-33-2) and customs tariff codes. This kind of clarity helps track down problems in case something goes wrong in distribution, and it builds trust between suppliers and big buyers.
Large-scale producers usually combine citric acid with a calculated amount of sodium carbonate or sodium bicarbonate. The reaction takes place in stainless steel vessels, with heating and stirring steps to keep things even. Engineers direct the solution through multiple filters to remove impurities, then concentrate the liquid under vacuum. The dissolved disodium citrate crystallizes on cooling, and the crystalline solids get washed and dried under carefully monitored conditions. Manual oversight steps in at QC checkpoints, ensuring that off-spec material gets separated before anything reaches packaging. Many producers recycle filtrate water and specialty by-products to cut environmental impact and improve efficiency.
Disodium citrate’s strongest feature comes from its buffering traits. It interacts easily with acids and bases, nudging any solution toward a stable pH. Soft drink factories use it to prevent wild swings in acidity, cutting risks when formulas include fruit extracts that change seasonally. It acts as a calcium chelator—binding up calcium ions, which is crucial for avoiding precipitation in certain applications. For laboratory use, it helps stabilize solutions during complex titrations or when handling sensitive reagents. Researchers often run derivatization reactions using the sodium or citrate parts as handles. Though it doesn’t react violently with other common food additives, some industrial chemists tweak the molecule by partial or full neutralization to get sodium-to-acid ratios perfect for niche needs.
Sodium hydrogen citrate stands as a common synonym, but you’ll also find nomenclature like E331(ii), disodium 2-hydroxypropane-1,2,3-tricarboxylate, and more simply, “buffering salt.” Warehouse labels in North America and Europe often refer to “disodium citrate dihydrate,” especially for forms containing bound water. For specific technical grades used in chemical synthesis or diagnostics, descriptors may feature variations like “analytical reagent grade” or “USP grade.” These differences matter in regulatory paperwork and when technical teams compare specifications across suppliers offering apparently similar material.
Handling disodium citrate follows safety policies standard in chemical industries. Workers use gloves and dust masks, given it can mildly irritate skin or eyes if handled carelessly. The compound does not come with the kinds of dramatic hazards seen with strong acids or bases, but bulk storage still takes place under dry, covered conditions to block moisture that could degrade product quality or encourage caking. Material Safety Data Sheets (MSDS) recommend safe disposal in compliance with local rules, since sodium and citrate ions both impact water chemistry at scale. Manufacturing plants run routine air quality and dust checks and train staff on how to minimize spills during production and packing. Reputable outfits hold certifications like ISO 9001 or FSSC 22000 and keep traceability records for every lot sold, which has become essential as global recalls in the food sector remind everyone of the value in strong documentation.
Disodium citrate finds its way into sodas, sports drinks, processed cheeses, and even baby foods because it softens flavors without masking them. Cheese makers depend on it to create meltable slices and spreads, since the compound binds up calcium and keeps fat and water in line during heating. Drug makers use it in antacids and effervescent tablets for acid control and to improve taste. Lab workers count on its buffering strength in biochemistry kits and diagnostic reagents. A few cleaning product brands throw it into laundry and dish detergents to bolster water softening without adding gritty residues. In the photography and plating industries, it acts as a sequestering or complexing agent. Healthcare settings sometimes rely on it in IV solutions to control blood acidity or as an anticoagulant, though those applications call for tight controls on purity.
Research teams dig into disodium citrate’s biocompatibility and its utility as a buffering agent for new medical treatments. Scientists work on optimizing crystal size and drying processes to improve the product’s mixing and dissolution behavior for finished foods and pharmaceutical blends. Some ongoing projects use it in controlled drug release tablets, aiming to modulate pH and drug solubility in the stomach or intestines. Teams specializing in green chemistry look for ways to produce the compound with lower energy consumption or fewer byproducts, using alternative raw materials like bio-based citric acid. In the diagnostic world, companies test it alongside novel bioreagents to create more stable, long-lasting test kits. With advances in process analytics, more producers rely on real-time monitoring with inline sensors and automated feedback loops to tighten up quality from the reactor right through to bagging.
Toxicologists studying disodium citrate report that it breaks down into sodium and citrate ions, both present naturally in the body’s metabolism. Animal studies and human use data reveal a reassuring safety profile at doses found in foods and medicines. At very high intakes, some people experience digestive upset from the sodium load. People with strict sodium limits or compromised kidney function face higher risks if exposed to large quantities, so food makers limit sodium citrate and its salts in low-sodium and renal-friendly products. Regulatory agencies like the FDA and EFSA set conservative maximum intake levels after studying how the body absorbs and excretes the compound. Occupational exposure limits have not been deemed necessary for most situations, thanks to its low acute and chronic toxicity.
Demand for disodium citrate will grow as more food developers look for clean-label souring and buffering options that carry a natural image. Its long record of safe use backs efforts to replace less familiar acid regulators in snacks, sauces, and functional beverages. Pharma firms expand its use in pediatric and geriatric medicines because of the mild taste and pH adjustability. Developments in fermentation could help make the production cycle cheaper and greener, cutting the industry’s dependence on fossil-fuel-derived chemicals. Bioplastics researchers now explore it as a stabilizing salt for new environmentally friendly polymers. Regulatory shifts, particularly in sodium intake guidelines, will push producers to refine blends that keep flavors sharp and shelf lives long without tipping over into unacceptable sodium levels. Research is likely to focus more on optimizing dissolution rates, reducing unwanted interactions, and unlocking extra roles in health-related and industrial applications.
Disodium citrate keeps popping up on ingredient lists for foods people eat every day. It’s a salt that steps up in juice boxes, processed cheese, lemon-lime sodas, and beyond. Beyond that, it has some important jobs in medicine cabinets and cleaning closets, too.
Taste isn’t the only reason disodium citrate lands in shelf-stable products. It plays referee for acidity, helping tangy drinks or dairy products hold a steady pH. Processed cheese gets its signature melt because disodium citrate prevents clumping. That’s something I learned years ago trying to make homemade cheese sauce—skip the citrate and you get oily, separated curds.
Controls like that mean companies can offer more consistent products. A study published by the Journal of Food Science found that using disodium citrate as an emulsifier changes not just texture, but keeps cheeses stretchy and sauces smooth for longer. With busy schedules and global shipping, nobody wants cheese that won’t melt or lemonade that upsets their stomach.
Energy drinks, soft drinks, and juice blends get another benefit from disodium citrate. It keeps flavors bright, but it also keeps certain bacteria from multiplying, especially in low-acid drinks. When foods last longer in the fridge or pantry, waste drops and families spend a little less. According to the FDA, disodium citrate helps keep shelf-stable drinks fresh without relying solely on extra sugar or harsh preservatives.
Stepping away from the kitchen, medical and pharmacy aisles rely on disodium citrate, too. Medicines for indigestion often use it as an antacid, soothing heartburn or sour stomachs. Emergency rooms use it in solutions like oral rehydration salts. For someone hit by dehydration from illness or heat—a real concern during long, hot summer hikes—drinks with disodium citrate make it easier for the body to absorb fluids.
Doctors sometimes recommend it for urine alkalinization, which helps some patients prevent painful kidney stones. These seemingly small benefits can make a world of difference for anyone prone to urinary tract infections or on certain medications.
My experience cleaning hard water stains made me rethink ingredients like disodium citrate. Its power to bind minerals means fewer spots on dishes and cleaner washing machines. Manufacturers of detergents and dish pods rely on this quality, especially where water runs hard, saving people from constant scrubbing and limescale worries. This helps appliances last longer.
The FDA classifies disodium citrate as generally safe when used properly, but that doesn’t mean “the more, the better.” Some people with certain kidney issues or on sodium-restricted diets should check labels or ask a doctor before regularly consuming foods high in additives like this one. Transparency matters—not just for safety, but for trust.
Manufacturers can keep refining their recipes, aiming for fewer extra additives and more transparent labeling. Consumers, empowered by more information, can choose what feels right for their families. Maybe most of us never expected an ingredient like disodium citrate to have such a broad reach, but modern life puts surprising compounds in places we wouldn’t expect.
You spot long, chemical-sounding words on food ingredient lists every day. One that pops up more often than you might realize is disodium citrate. It works like a buffer or acidity regulator in soft drinks, powdered mixes, cheese, jelly, and even some medicine. It helps food taste right and last longer, so it ends up in plenty of processed snacks.
Looking at what’s tucked into our food, I like to ask, “Will this actually cause harm in my kitchen, or am I just reading chemistry class flashbacks?” With disodium citrate, there’s plenty of history to look at: its parent compound, citric acid, shows up naturally in citrus fruits and tomatoes. Disodium citrate comes from this same stuff, just tweaked for food-processing needs. It lets manufacturers control tartness and preserve flavors without throwing off other food qualities.
The U.S. Food and Drug Administration classifies disodium citrate as GRAS (Generally Recognized as Safe). It’s approved for use in Europe and goes through regular safety reviews at international health organizations, including the Joint FAO/WHO Expert Committee. These groups look at how people eat, drink, and digest this additive and they agree—at the levels used in foods, it doesn’t bring up red flags for healthy adults.
I've mixed sports drinks after workouts and reached for processed cheese to make quick lunches for my kids. If you read the label, disodium citrate pops up in both. Most people only take in small amounts daily. Studies point out that the human body knows how to handle it—it converts it into harmless citric acid, then flushes it out naturally.
Large-scale studies just haven’t linked regular food-grade use to kidney issues or stomach irritation. Now, extremely high doses (far beyond food levels) given for medical reasons have caused concern for people with chronic kidney conditions, but that’s in a hospital setting. People with rare sodium-handling issues or kidney trouble ought to talk with their doctor, especially if taking sodium-containing medications along with processed foods.
Often, safety isn’t about one ingredient. Instead, it comes down to eating habits. Processing makes foods last but can also pack in sodium and other additives, which add up if your diet relies heavily on packaged snacks, sodas, or pre-cooked meals. That doesn’t mean disodium citrate itself is the main concern—instead, look at the whole food, how many processed foods you’re eating, and where sodium totals wind up in your daily routine.
Knowledge helps more than guesswork, so keep reading labels. I always encourage moderation and more fresh foods to balance out the easy options loaded with sodium and preservatives. Cooking simple meals at home using whole ingredients naturally cuts back on additives, without resorting to paranoia or cutting out everything convenient.
Disodium citrate doesn’t fall on the “beware” list for most people. If you’re healthy, there’s no evidence these levels act any differently than salt or natural citric acid. Anyone managing kidney issues or high sodium intake from other sources should bring that up during a doctor’s visit, to get advice based on their own health reality. Rely on doctors and scientists, not rumors, for peace of mind about what lands on your plate.
Disodium citrate pops up in quite a few places: hospitals use it in medicines, food producers add it to processed cheese and sodas, and even some home brewing fans swear by its pH-balancing power. With this much use, people sometimes forget that every additive can bring side effects, especially when the dose creeps up or someone’s health makes them more vulnerable. Working in health communication, I’ve seen both casual users and patients discover these effects the hard way.
Digestive discomfort shows up the most often. People tell me about bloating, burping, or a stomach twinge after taking a remedy that includes disodium citrate. The science backs this up—excess alkalinity can disrupt the stomach’s balance, leaving some folks with a sour stomach or reflux. You might also see a laxative effect if you take more than recommended amounts. That quick trip to the bathroom isn’t just in your head; this compound pulls water into the bowel, leading to loose stools.
Mild headache or thirst also gets reported. That’s because sodium in the body, even in moderate doses, can shift how much water your tissues hang onto. Anyone with salt-sensitive blood pressure should take note—raising sodium intake may inch blood pressure higher, which doesn’t benefit anyone already taking meds for hypertension.
Most people won’t face severe problems if they stick to recommended amounts, but exceptions pop up. Chronic kidney disease patients can’t filter sodium and citrate as well as others. Their bodies hold onto more, which can lead to swelling, fluid overload, and even heart rhythm issues. Metabolic alkalosis, where the blood swings more alkaline than it should, sounds rare, yet I’ve seen hospital patients tip into this state after regular doses for indigestion. The symptoms—confusion, shaking hands, cramping—lead doctors straight to the labs.
Those with heart disease need to tread carefully, too. Extra sodium bumps up strain on their cardiovascular system, and for some, that means a spike in blood pressure or even abnormal heart rhythms.
Reading labels and understanding your own health history gives you a real advantage. Folks on blood pressure meds or with kidney conditions benefit from talking to their doctor before adding over-the-counter products with disodium citrate. Sometimes safer alternatives exist, and pharmacists can guide that choice.
Better education helps. When working with patients, I explain why that “harmless” antacid or cheese spread might not suit everyone. If you feel off after using these products—thirstier, tired, or noticing digestive symptoms—stop and check in with a healthcare provider. Side effects aren’t just rare warnings; they become real-life hassles for certain people.
The food and supplement industry continues to lean on additives for a consistent product, but healthcare needs to keep an eye on those with pre-existing conditions. Careful usage, honest conversations with professionals, and a bit of label-reading go a long way. No one should learn about side effects only after they’ve become a reality.
If you’ve ever taken a heartburn remedy, you may have seen disodium citrate listed on the label. This salt draws a lot of attention in both food and medical circles. It’s used to control acidity, to act as a buffer, and, in medicine, to help relieve mild urinary discomfort by changing the pH balance. Disodium citrate stands out because of its safety track record when handled correctly. Like most ingredients, it is all about the dosage; taking more than needed does not improve its benefits, and taking too much can be risky.
Let’s get straight to it. In food, disodium citrate plays a key role as an acidity regulator, often found in cheese spreads, soft drinks, and certain baking products. The U.S. Food and Drug Administration (FDA) tags it as “generally recognized as safe” (GRAS), but that status isn’t a green light to dump unlimited quantities into food. Manufacturers usually keep the dose between 0.2% to 0.5% by weight of the finished product for taste and preservation, making sure not to overdo it.
When people take disodium citrate as a medicine for urinary alkalinization or as an ingredient in some antacids, the numbers look different. Most products recommend 1 to 3 grams, diluted in water, taken up to three times per day. This is not something to guess at. Most adults can handle doses in this range, but people with kidney problems, heart issues, or high blood pressure should speak to a doctor first. In children, only a pediatrician should set the dose, usually much lower than in adults.
Through decades of seeing patients, I’ve noticed how often people assume that double the amount brings double the relief. That’s rarely true. Disodium citrate alters the body’s sodium load and fluid balance. Large doses put a person at risk for high blood pressure, fluid retention, or, in rare cases, metabolic alkalosis (when the blood becomes too alkaline). These outcomes show up more in someone with an underlying health issue or in kids, who are less able to compensate for electrolyte shifts.
The body already works hard to keep sodium and potassium levels balanced. A supplement like this, in excess, can tip the scales and create more trouble than it solves. The European Food Safety Authority (EFSA) reviewed the data and found no need for a specific “tolerable upper intake level” in food because typical use levels stay so low. But as a supplement or medicine, sticking with labeled directions is crucial.
If in doubt, check the product instructions or ask a pharmacist. Some people hear about potential health “hacks” online and may try to self-dose for kidney stones or other urinary tract issues. That is never safe. Those with high blood pressure, chronic kidney disease, or pregnancy shouldn’t use disodium citrate unless cleared by a physician. The margin between a helpful dose and a harmful one can be slim, depending on personal health.
Government agencies and large health organizations base their recommendations on research, not hearsay or product marketing. Manufacturers must run regular product checks to make sure every scoop or pill matches the intended amounts. If you’re choosing to use disodium citrate, for food or health reasons, do so with information and common sense as guides.
Disodium citrate often sits quietly on ingredient lists and in over-the-counter meds. Its main job is to regulate acidity and act as a buffering agent. From fizzy drinks to certain chewable tablets, this salt works behind the scenes. But it’s more than a background player in some situations. People dealing with kidney stones or certain metabolic troubles may find it prescribed to keep their urine less acidic.
Mixing medications poses a real risk, especially when the kidneys and the body’s chemical balance are involved. Anything that alters acid-base levels can nudge other drugs into new, sometimes unpredictable directions.
Certain antibiotics and heart medications have shown potential for interaction if disodium citrate comes into the picture. For example, antibiotics like tetracyclines or fluoroquinolones may see their absorption reduced if stomach acid gets altered. Blood pressure medicines, particularly those that affect potassium, sometimes do not mix well with agents that shift pH or electrolyte levels. Even antacids featuring aluminum or magnesium can run into problems—acid changes might enhance or limit how these medicines work.
Disodium citrate isn’t guilty by default, but once it enters the body, changes start happening. People with chronic kidney disease or heart problems might experience a build-up of sodium or trouble with fluid retention. Folks taking water pills or drugs that protect the heart should take note, since sodium levels and blood pressure may swing out of balance.
Some experts have documented cases where increased alkalinity from citrate compounds made it harder for certain medications to dissolve or get absorbed. While hospital teams routinely check electrolyte levels, regular folks relying on over-the-counter products may not realize what’s at stake.
No one wants a simple antacid or citrate supplement to derail their treatment plan. Anyone juggling long-term prescriptions—like anti-epileptics, diabetes drugs, or immunosuppressants—should ask a pharmacist before picking up something new at the pharmacy. Doctors can adjust other medications if they know about the addition, and pharmacists check for well-known risks using drug databases.
Working in a hospital setting, I’ve seen cases where a simple antacid led to changes in a patient’s potassium or calcium levels, causing muscle cramps or even cardiac trouble. That’s a serious outcome for such a common additive.
Read medication labels and don’t skip any over-the-counter products during medication reviews. Keep a running list of every supplement, prescription, or standalone ingredient—even if it seems harmless. Asking a pharmacist or doctor about drug interactions should be second nature, especially for elders or people with multiple health conditions.
Pharmacies never mind a question like, “Does this work well with my current meds?” Clinical guidance points to open dialogue and routine checks for everyone on long drug lists. Health professionals have access to updated resources tracking these interactions and can spot trouble before it starts. That’s an easy habit that saves plenty of headaches and, sometimes, lives.

| Names | |
| Preferred IUPAC name | disodium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Disodium hydrogen citrate Disodium dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate E331(ii) |
| Pronunciation | /daɪˈsəʊdiəm ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 144-33-2 |
| 3D model (JSmol) | `JSmol.loadInline("data/mol:disodiumcitrate")` |
| Beilstein Reference | 1720489 |
| ChEBI | CHEBI:40263 |
| ChEMBL | CHEMBL1201188 |
| ChemSpider | 52165 |
| DrugBank | DB09122 |
| ECHA InfoCard | ECHA InfoCard: 03-2119969285-26-0000 |
| EC Number | 200-675-3 |
| Gmelin Reference | 9244 |
| KEGG | C00735 |
| MeSH | D003377 |
| PubChem CID | 6224 |
| RTECS number | GE8300000 |
| UNII | K848J56Y48 |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID3023497 |
| Properties | |
| Chemical formula | Na2C6H6O7 |
| Molar mass | 294.10 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.8 g/cm³ |
| Solubility in water | 642 g/L (20 °C) |
| log P | -3.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa1 = 3.13, pKa2 = 4.76, pKa3 = 6.40 |
| Basicity (pKb) | 0.17 |
| Refractive index (nD) | 1.47 |
| Dipole moment | 2.60 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 302.4 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1560.6 kJ/mol |
| Pharmacology | |
| ATC code | B05CX02 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. |
| GHS labelling | GHS labelling: "Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008 [CLP/GHS]. No pictogram, signal word, hazard or precautionary statement required. |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008 [CLP/GHS]. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 210 °C |
| Autoignition temperature | > 745°C |
| Lethal dose or concentration | LD50 (oral, rat): 8,200 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 8200 mg/kg |
| NIOSH | WH3400000 |
| PEL (Permissible) | 15 mg/m³ |
| Related compounds | |
| Related compounds |
Monosodium citrate Trisodium citrate Citric acid |