Ethyl lactate first came onto the scene through organic synthesis, popping up as a compound of interest for its roots in renewable resources like corn and other plants. The chemical world paid little attention at first, seeing it as just another ester, but as environmental regulations tightened in the late 20th century, chemists started looking for non-toxic, biodegradable substitutes for harsh petrochemical solvents. Ethyl lactate began getting respect for its plant-derived origin and gentle environmental profile, offering relief for industries desperate to move away from aggressive, lingering solvents.
Getting to know ethyl lactate starts with its background as the ethyl ester of lactic acid. This combination delivers an attractive blend of good solvency and easy biodegradability. Bottles and barrels labeled “ethyl lactate” now contain a chemical with a fruity scent that works almost anywhere—electronics cleaning, paint thinning, and even some food-grade processes. Its growing demand reflects a society hunting for greener alternatives without sacrificing the quality expected in manufacturing, pharmaceuticals, or even niche cosmetics.
Ethyl lactate presents itself as a clear, colorless liquid, recognizable by a subtle, sweet aroma not far from green apples or freshly-cut pears. Its boiling point rests around 154°C, showing enough thermal stability for most industrial tasks, and at room temperature, it shines with miscibility in water and most organic solvents. Its chemical make-up, C5H10O3, opens many doors for reactions—thanks to both an ester group and a hydroxyl function. Density stays close to 1.03 g/cm3, and its flash point at roughly 46°C calls for some care in handling but doesn’t match the volatility of traditional, petroleum-derived ethers or ketones.
On shelves and in online catalogs, ethyl lactate shows up with technical specs that highlight purity (often exceeding 98%), alongside metrics like water content (limited below 0.5%) and acidity. Labels draw on standardized identifiers: CAS number 97-64-3, EINECS 202-598-0, and proper chemical names. Packaging usually meets regulatory frameworks that set out by the EPA and OSHA, requiring clear hazard designations and guidance on personal protective equipment. Manufacturers stamp out batch numbers, storage instructions, and expiration dates, which matters for quality auditing or if something ever goes sideways in the supply chain.
To craft ethyl lactate, most facilities lean on direct esterification. Chemists react lactic acid—usually from fermented biomass—with ethanol, and the process slides along with an acid catalyst in a controlled reactor, sometimes using azeotropic distillation to drive the water off and push the reaction to completion. Some smaller labs might use more delicate purification, but for industrial quantities, robust distillation keeps the flow going. This method rests on the availability of feedstock—a world awash in corn syrup and plant sugars grants the process a sustainability edge over solvents built from fossil fuels.
Ethyl lactate’s chemical structure does more than earn it a spot in green chemistry. Under acid or base conditions, it hydrolyzes back to lactic acid and ethanol—so any residue that winds up in wastewater treatment plants doesn’t stay a pollutant. Chemists tweak the molecule through transesterification or oxidation, building out new specialty esters or even using it as a mild reaction medium to coax sensitive transformations along. Its blend of reactivity and stability means it works as both raw material and safe carrier for many active pharmaceutical ingredients.
Anyone shopping for ethyl lactate will bump into an assortment of aliases: lactic acid ethyl ester, d(-)-lactic acid ethyl ester, or green solvent. Commercial and technical sheets add even more flavor, with products sold as “GreenSol EL,” “PURASOLV EL,” and “Ethyl-Lact.” This parade of names sometimes pushes labs and purchasing managers to double check they actually get the biodegradable ester they want, not a substituted cousin with a less-friendly profile.
Handling ethyl lactate means knowing its limits: low acute toxicity but enough flammability to demand good ventilation and spark-free storage. The chemical has earned approval under many green chemistry programs, but facilities stick to gloves, goggles, and NIOSH-approved filtration masks for extended work. Spill management leans toward quick absorption and removal—its mild organic profile keeps response teams calm compared to old-school, high-toxicity solvents, though it still poses risk if left pooled in confined spaces. OSHA and REACH regulations lay out baseline safety, with clear cut-off values for permissible exposure that line up with the solvent’s benign status.
Ethyl lactate now powers a big swath of industry, from paint thinners to cleaning circuits in the semiconductor world. Printers use it as a green alternative for ink removal, electronics refineries rely on it for flush-and-clean routines, and pharmaceutical operations swap out more hazardous options for its friendly profile. Even the food industry dips its toe—a few niche flavor extractors find value in its low toxicity and gentle touch, especially when making natural aromas from botanicals or spices. Conservation labs handling delicate art pieces celebrate it for stripping away old varnish without leaving toxic residue in museum storerooms.
Universities and chemical companies keep the pipeline moving forward, testing ethyl lactate in everything from drug delivery to polymers that break down more easily in the wild. Recent journals include studies on its effectiveness as a solvent for biopolymers and degradable plastics, as well as investigations into blending this ester with newer ionic liquids for broader compatibility. Industry partnerships between solvent manufacturers and consumer goods titans often emerge, hinting at a future where common cleaning sprays, adhesives, and packaging swap petroleum for more plant-based chemistry.
Over years of study, toxicologists agree that ethyl lactate carries a low risk for acute toxicity. Standard rat studies place the oral LD50 upwards of 5,000 mg/kg, which puts it far from the danger zone for daily contact. Most irritation worries center on high concentrations in direct skin or eye exposure, where the ester’s mild acidity can bring on redness or discomfort, but nothing life-threatening. Workers handling it full-time show minimal systemic absorption, and breakdown byproducts match what the body sees from metabolism of lactic acid during tough workouts or digestion of fermented food. As a bonus, breakdown products don’t linger in water or soil, and regulatory agencies in Europe and the US classify ethyl lactate as a low concern for environmental persistence.
As green chemistry gathers steam, ethyl lactate looks set for wider adoption—both through regulation and consumer pressure. Lab researchers now toss this solvent into test runs with biodegradable plastics, new drug carriers, and safer industrial coatings. Policy shifts in the EU and North America push cold-start manufacturers to reconsider legacy solvents, and many find ethyl lactate’s steady supply chain and price point finally matching its established rivals. Growing global concern over microplastics and persistent toxins has sunlighted solutions like ethyl lactate, motivating more universities, startups, and chemical giants to lean in, seek better methods, and launch new product lines that swap out the chemical rot of the last century. And as plant-based feedstocks become more efficient, the cost, carbon footprint, and technical barriers of using ethyl lactate continue to shrink—paving the way for a world where friendly chemistry leads not with profit, but with purpose.
Ethyl lactate comes from lactic acid and ethanol, two things you often find in foods and drinks. This liquid smells faintly sweet. It’s not some chemistry-class relic. Instead, it shows up in your life more than you might realize. I first ran into ethyl lactate in college, working a part-time janitor gig. The boss switched the cleaners out for this clear liquid and called it a “new green solvent.” I barely knew what that meant, but after long shifts scrubbing paint off floors, I noticed something: this stuff cut through grease as well as the old-school solvents but didn’t set off headaches or stink up my clothes. Years later, I realized I had seen the beginnings of a bigger change.
Ethyl lactate comes into play for people working in cleaning, painting, and even food processing. Paint shops use it to thin coatings. In lithography and electronics, folks need to scrub away tiny residues after making circuits. Most thinners leave the workspace smelling like chemicals for hours, but ethyl lactate breaks down in air and water more easily, helping reduce some of that chemical fog. In food factories, workers rely on ethyl lactate for degreasing equipment that handles oils or dairy. The U.S. Food and Drug Administration considers it safe enough for these areas, so a batch of pastries won’t take on strange flavors.
Chemical safety makes a difference beyond what you smell. Harsh petrochemical solvents—like acetone or toluene—carry risks for people handling them every day. Regular exposure can mean rashes, headaches, or serious nerve damage in the long run. Ethyl lactate, pulled from ingredients already passing through nature, offers fewer hazards while doing the same tough work. It breaks down to harmless molecules much faster outside a factory. That matters for workers rinsing tools right into city drains and for anybody living near industrial sites. Wastewater flows cleaner, and there’s less residue hanging around. Research backed by the EPA and peer-reviewed journals lays this out: tests show ethyl lactate decomposing in soil or water with the help of bacteria rather than lingering for years like many old-school solvents.
Big switchovers always run into bumps. Ethyl lactate is pricier than some petrochemical alternatives, especially if companies buy in bulk. That gap shrinks as demand rises and production scales up, but cash-strapped outfits still hesitate. In my experience, explaining short-term costs versus long-term health savings helps. Fewer worker accidents or lawsuits cut expenses later. Some users point out that ethyl lactate pulls water from the air, so it may not suit every process. In paint shops or labs, people sometimes need to tweak formulas so the results match what they depend on. Research and development teams experiment with mixtures and combinations. Universities and government grants play a role in finding better ways to use it, lower production expenses, and avoid trade-offs in performance.
Ethyl lactate’s story isn’t about pushing a single miracle cure—it’s proof that older habits can evolve with a focus on safety and responsibility. Community voices, solid science, and feedback from the people using these chemicals daily keep the conversation alive. No quick fixes, but steady change shows real results, both at work and beyond.
Ethyl lactate pops up in conversations about green chemistry, especially among people swapping out old-school solvents. People call it “bio-based” since you get it from the fermentation of corn sugar. That’s a strong selling point in a market where petrochemical solvents have a bad track record. Corn grows in fields, not oil rigs. It’s a renewable resource that many believe supports a circular economy, at least in theory.
Farming corn for industrial use marks a shift from the chemistry lab back to the soil. Plenty of folks see this as a step forward—less reliance on fossil fuels, more support for rural economies, possible reduction in global supply chain pressure. Recent USDA data shows the U.S. churned out 15.3 billion bushels of corn in 2023, making the production of ethyl lactate on a larger scale feasible. But it’s not all perfect—heavy fertilizer and pesticide use in corn farming brings its own troubles, like water runoff and soil health risks.
One of the good things about ethyl lactate: it breaks down pretty quickly in the environment. Studies from the EPA note that it’s “readily biodegradable.” Pour it on the ground or spill it in water, and naturally occurring microbes finish the job fast. The breakdown products—lactic acid and ethanol—don’t stick around and cause long-term headaches like halogenated solvents.
People working in labs often say it smells like green apples. More importantly, it doesn’t give off the harsh fumes associated with toluene, acetone, or similar solvents. That makes it easier and safer to handle for people on the job every day. In my own experience working with different chemicals, I’ve seen how a less-toxic solvent makes a big difference for workers’ comfort and for the air in the workspace.
Turning corn into solvent takes energy. Growing corn calls for tractors, irrigation systems, transportation, and then you’ve got the actual fermentation and distillation at the factory. Some plants run on renewables, but plenty still use coal or natural gas. The environmental benefits of switching to ethyl lactate get cut down when the production side leans on fossil fuels.
Comparing it to petroleum-based solvents, some studies point to a 30–40% smaller overall carbon footprint, but these studies often assume best-case farming and processing conditions. If demand for ethyl lactate grows, corn acreage expands, energy use rises, and so does the environmental cost.
Ethyl lactate isn’t a silver bullet. Large-scale production leans on industrial agriculture, which impacts biodiversity, water tables, and local communities. And if the world keeps pushing for plant-based everything without changes in farming and factory energy sources, the environmental gains could evaporate.
Looking ahead, growing more corn using regenerative farming, switching to wind and solar in processing, and tightening regulations could tilt the balance. Reducing single-use plastics and fossil-derived solvents will take more than one molecule. Ethyl lactate offers a cleaner alternative for working chemists and factories, but real environmental change calls for a whole-system approach—from the field to the factory and back to the earth.
Plenty of companies now list ethyl lactate as a safer, greener choice in their cleaning products, inks, and coatings. Choosing it over old standbys like NMP or xylene cuts exposure to dangerous chemicals and sidesteps some waste stream woes. That said, businesses and policymakers can’t just check the “bio-based” box and call the job finished. They need to keep asking tough questions: Where does the corn come from? What powers the plant? What happens downstream? Otherwise, “green” marketing turns into just another sticker on the bottle.
Ethyl lactate pops up in all kinds of settings: printing shops, paint booths, and even food processing plants. It’s considered safer than many older solvents, partly since it comes from corn and is biodegradable. But being from a “natural” source doesn’t guarantee safety with every use. Plenty of folks assume low toxicity equals no harm—until they end up with a headache, rash, or worse. Ethyl lactate evaporates fast, giving off a sweet aroma that tricks people into thinking it’s harmless. In reality, repeated or poorly ventilated contact paints a different story.
Breathe in those vapors for any length of time, and it’s not just your nose and throat that notice. Early in my chemistry career, I skipped on gloves once when cleaning lab glassware. Not long after, my hands felt dry and itchy, and the smell lingered even after several washes. Folks who handle the stuff without eye protection sometimes squint or deal with irritation. In industries where spills aren’t rare, even one slip can turn a regular day into a frantic cleanup. These honest mistakes underline why safety details matter, no matter how busy the schedule looks.
Direct contact causes skin irritation for some, so gloves made from nitrile or neoprene come in handy. Latex gloves often break down over time, so reaching for tougher material can prevent a quick soak-through. Safety goggles keep splashes out of the eyes, which matters because the solution can sting much more than you’d expect. In spots where splash potential rises—like drum transfers—face shields add another layer.
Getting a whiff of ethyl lactate isn’t the same as handling stronger chemicals like toluene, but ventilation helps every time. Opening windows, running exhaust fans, or working inside a fume hood drops exposure. I’ve seen shops save on costly medical bills just by upgrading air flow. For those mixing batches, spilled solvent on the skin should get washed away with plain soap and water right away. Water first, then soap, and repeat until there’s no sticky feeling left. Rubbing alcohol or other chemicals only complicate things, which manufacturers warn against for a reason.
Ethyl lactate might seem stable, but warm conditions and open containers easily kick off strong smells. Tightly sealed bottles stored away from heat sources and direct sunlight slow down evaporation and keep the air clearer for everyone. In my family’s garage, a forgotten, unsealed jar made the whole corner smell odd for a week. That mistake would shut down operations in a commercial setting where regulations stand watch.
Bigger spills require more than just paper towels. Absorbent pads or sand work best for soaking up puddles, and gloves are always the right call during clean up. Used materials shouldn’t go straight into regular trash bins. Following local hazardous waste guidelines keeps landfills safer and prevents bigger environmental problems down the road. Even if it takes a few more steps, the payoff comes in fewer injuries and less trouble for the whole community.
Hands-on guidance makes all the difference. Safety sessions that walk through both small and big accidents help everyone make smarter decisions—especially when new hires join the crew. Tutorials and signs in the right spots, like near sinks and storage areas, refresh old memories and reach folks who move between jobs. Questions and small tests help measure real understanding, not just another signature on a form. It’s a habit that’s stuck with me ever since I started out, and it keeps surfacing every time new chemicals come into play.
Bottom line: Smart handling keeps everyone’s day on track. Ethyl lactate isn’t the villain of the chemistry cabinet, but shortcuts in safety add up. Gloves, airflow, quick washes, and solid training go further than a long list of rules. Most accidents look preventable in hindsight, so upfront care matters even more.
People hear "biodegradable" and instantly think of pristine rivers and clean air, but the real test comes from what science and experience say. Ethyl lactate stands out in many green chemistry conversations. It’s a solvent that doesn’t look like an enemy of the earth. It comes from renewable sources—corn or other sugary crops—so it doesn’t rely on fossil fuels as many traditional industrial solvents do.
A handful of years spent cleaning lab glassware and degreasing machine parts hammered home the difference between solvents. Petroleum-based cleaners linger everywhere: on skin, in water, in the air. Ethyl lactate never produced that stubborn odor or residue. It broke down faster, both in the drain and according to the paperwork from the supplier.
This isn’t just marketing. Ethyl lactate has a straightforward structure. Bacteria and fungi in soil or wastewater treatment plants can chew through it with ease, converting it mostly into lactic acid and ethanol, both familiar molecules in biology. Studies back this up: In typical aerobic conditions, ethyl lactate doesn’t last very long. If you poured some down the sink, the local microbial army could tackle it without a problem—usually within a couple of weeks, not months.
Why does this matter? Old-school solvents stick around, causing trouble from groundwater pollution to smog. I’ve seen spill cleanup crews spend days sucking up methyl ethyl ketone or xylene, and they always wore heavy-duty masks for a reason. Ethyl lactate, despite doing a similar cleaning job, brings much less risk after disposal. The solvent’s low toxicity for fish and water bugs gets highlighted by the European Chemicals Agency and the US Environmental Protection Agency. Low persistence plus low toxicity equals less long-term hazard for waterways and soil.
The green label doesn’t mean a free pass. Biodegradability depends on the environment. If ethyl lactate hits an anaerobic landfill without enough microbes or oxygen, breakdown slows down. Cold climates also tap the brakes on degradation. It can’t solve every pollution headache—spilling drums near drinking water sources still spells trouble.
Manufacturers also need to be careful about what else goes into their products. Adding heavy metals, chlorinated compounds, or dyes can turn an otherwise harmless solvent into a greater threat. This can turn a simple solution into a complicated waste stream. Checking the full list of chemicals in any industrial cleaner or paint ingredient stays smart practice.
Companies keep switching to ethyl lactate for good reason. Regulations in Europe and some US states push for safer, greener solvents. It helps to train employees so they understand what makes one cleaner safer than another and what to do if a spill happens. Wastewater treatment operators often look for substances that won’t choke microbes or throw off biological filters. Ethyl lactate fits the bill better than most petroleum alternatives.
Environmentally responsible use comes down to using the right tool for the job and knowing where it ends up. Ethyl lactate won't cure all ecological concerns, but in the ongoing puzzle of industrial chemistry, it brings a piece that fits a little more snugly than many of its cousins.
Over the last decade, more folks involved in food production have taken a close look at ethyl lactate. This clear liquid comes from renewable resources, namely corn and sugar beets, making it stand out from many traditional chemical options. Its clean scent reminds me a bit of green apples, not the harshness you get from industrial solvents. That’s probably one reason it caught my attention during a tour of a specialty food plant — it felt like a relief compared to the usual burn in my nose from some other chemicals.
Ethyl lactate jumps through some important safety hoops. The US Food and Drug Administration recognizes it as Generally Recognized As Safe (GRAS) for use as a flavoring agent. In Europe, it holds its spot on the authorized food additives list under the code E325. At home, I checked ingredient labels on some shelf-stable sauces and found it right there next to citric acid. Seeing a regulator’s blessing helps, but real-world handling matters too. Even with GRAS status, concentration levels and application style need careful management.
I’ve spoken with chemists who appreciate how ethyl lactate breaks down into simple lactic acid and ethanol, both common in fermented foods and drinks. Neither compound raises a red flag in moderation. As with salt or sugar, it’s a question of “how much” instead of “if it belongs at all.”
Food processing sometimes needs solvent power to pull flavors from botanical ingredients or to help dissolve sticky fats. Ethyl lactate does the job well without bringing along the baggage of chlorinated or petroleum-based solvents. In one flavor lab, I watched how it pulled delicate compounds from vanilla beans to give a fuller taste without overpowering the nose. It’s also made cleaning stainless steel equipment less painful, with no cloud of toxic fumes sending workers outside every hour.
That being said, using ethyl lactate means monitoring for residues, just like with any process aid. A slip in handling or poor rinsing could cause issues for both food safety and taste. Equipment needs good flushing and careful measurement at every step.
Switching to ethyl lactate across a whole facility could hit a few speedbumps. It’s more expensive than old-school solvents, so smaller operations balk at the price tag. Consistency is another sticking point. Since it’s sourced from crops, changes in harvests can affect purity or supply. I’ve heard from a few craft producers who’ve had to find backup options during harvest shortages.
To move forward, more research on residue limits and long-term impacts makes sense. Regular audits also add an extra layer of confidence for both producers and consumers. Governments and industry groups could step up to provide clear guidelines on processing aids like this one. If suppliers keep up transparency and regulators set clear, science-backed limits, ethyl lactate could become a staple for food makers interested in cleaner, greener operations. That would give both workers and eaters one less thing to worry about.
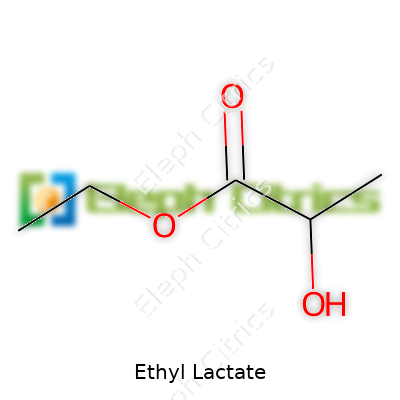
| Names | |
| Preferred IUPAC name | Ethyl 2-hydroxypropanoate |
| Other names |
Ethyl 2-hydroxypropanoate Ethyl α-hydroxypropionate Ethyl lactic acid |
| Pronunciation | /ˈiːθɪl ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 97-64-3 |
| 3D model (JSmol) | `ETHYL LACTATE; JSmol 3D model string:` `CCOC(=O)C(O)C` *(This is the SMILES string representation for use with JSmol to generate the 3D model of Ethyl Lactate.)* |
| Beilstein Reference | 607873 |
| ChEBI | CHEBI:27750 |
| ChEMBL | CHEMBL1359 |
| ChemSpider | 74896 |
| DrugBank | DB02754 |
| ECHA InfoCard | 03e27b88-5fd1-40e8-9e89-6384933b6bdc |
| EC Number | Ethyl Lactate" EC Number is " ethyl lactate: 211-063-1 |
| Gmelin Reference | Gmelin Reference: **8365** |
| KEGG | C02710 |
| MeSH | D018380 |
| PubChem CID | 7322 |
| RTECS number | KO3225000 |
| UNII | N1Q0FLO5QT |
| UN number | UN1192 |
| Properties | |
| Chemical formula | C5H10O3 |
| Molar mass | 118.13 g/mol |
| Appearance | Clear, colorless liquid |
| Odor | fruity |
| Density | 1.03 g/cm³ |
| Solubility in water | Miscible |
| log P | -0.24 |
| Vapor pressure | 0.46 mmHg at 25 °C |
| Acidity (pKa) | pKa = 15.3 |
| Basicity (pKb) | 11.55 |
| Magnetic susceptibility (χ) | -6.58 × 10⁻⁶ |
| Refractive index (nD) | 1.414 |
| Viscosity | Viscosity: 2.5 cP (at 25°C) |
| Dipole moment | 4.26 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 168.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | −674.2 kJ·mol⁻¹ |
| Std enthalpy of combustion (ΔcH⦵298) | -1334.1 kJ/mol |
| Pharmacology | |
| ATC code | V07AB |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H319 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P312, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | '1-2-0' |
| Flash point | 54 °C |
| Autoignition temperature | 225 °C |
| Explosive limits | 1.5% - 8.5% |
| Lethal dose or concentration | LD50 (oral, rat): 5,000 mg/kg |
| LD50 (median dose) | LD50 (median dose) = 5,000 mg/kg (oral, rat) |
| NIOSH | KKG379 |
| PEL (Permissible) | PEL: 25 ppm |
| REL (Recommended) | 5 mg/m³ |
| IDLH (Immediate danger) | 1000 ppm |
| Related compounds | |
| Related compounds |
Lactic acid Methyl lactate Butyl lactate Propylene glycol Ethyl acetate |