Through the ups and downs of chemical industry progress, ethyl octanoate has kept turning up as a flavor ingredient worth remembering. Decades back, chemists cracked its structure using some pretty basic distillation and esterification techniques. Long before food chemists started to drool over "natural" and "artificial" distinctions, winemakers and perfumers stumbled across ethyl octanoate’s fruity allure. It first showed up, documented in wine and spirits, when researchers tried to pin down what made fruit brandies so aromatic, way back in the early twentieth century. The rise of modern food science during post-war industrialization paved the way for synthesizing the compound at larger scales. As industries looked for ways to mimic or boost natural flavors, ethyl octanoate transformed from a curious molecule into a workhorse of the flavor and fragrance world.
Anyone who has sipped a glass of white wine or peeled a ripe apple has brushed up against ethyl octanoate. Commercial producers look for bright, persistent fruity notes, and this compound delivers. It often comes as a colorless liquid, packing a powerful scent that drifts between pineapple, pear, and a tinge of rum. Companies sell it in drum quantities, shipping it to blenders who want to boost the appeal of everything from soft drinks to fancy perfumes. Buying it pure or in custom blends is all about the batch size and downstream use. Food and beverage companies source food-grade quality while fragrance firms lean on high-purity versions. Price and supply shift a bit with global economic changes, but ethyl octanoate remains a staple for anyone needing a reliable, natural-smelling fruit flavor.
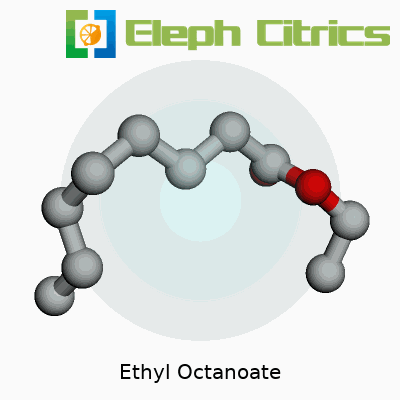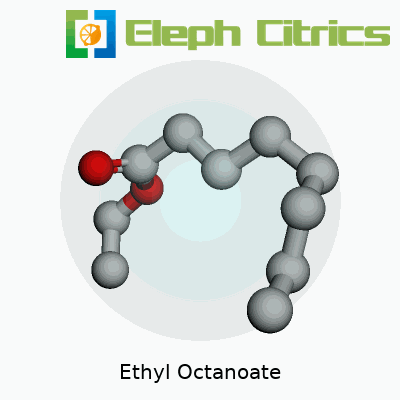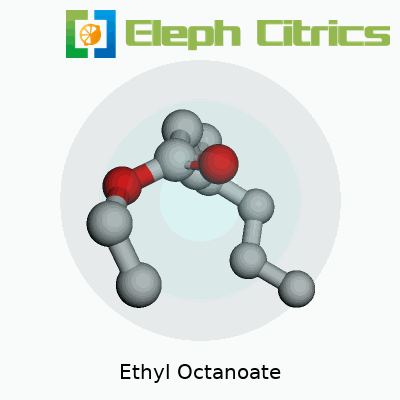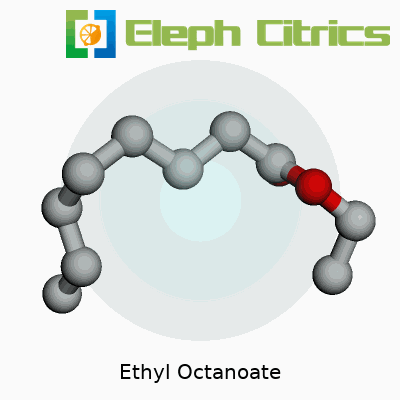
Ethyl octanoate belongs to the family of esters, known for their sweet, often floral scents. Its chemical formula stands at C10H20O2, and its molar mass clocks in at 172.27 g/mol. The liquid doesn’t linger with a heavy hand; boiling point sits around 210°C, and it carries a low melting point, just below normal room temperatures. The compound barely mixes with water but blends well with ethanol, ether, and all sorts of organic solvents. That makes it both useful and, sometimes, a challenge for folks handling large batches—careless storage can lead to evaporation losses. On the tongue, it brings a creamy, smooth quality, which explains its role in dairy and candy flavors. Its flashpoint of around 87°C also reminds handlers to keep fire safety at the back of their minds during transport and storage.
Technical sheets for ethyl octanoate lay out standards for appearance, odor description, purity (often above 98%), and limits for impurities like water or residual solvents. Food-grade labels insist on compliance with globally-recognized rules, with certificates showing the absence of heavy metals and confirmed allergen statements. Shipping labels must flag the product as flammable and spell out proper UN numbers for global trade. For every industry buyer, batch traceability and quality-control results drive purchasing decisions. Companies serious about compliance want GC-MS printouts and analyses that tie back every drum to a production lot. Any gaps in specs or missing labels risk regulatory headaches or product recalls—no one wants to explain a fruity off-note or a mislabeled batch to downstream clients.
Lab chemists and factory engineers both rely on esterification, the classic reaction between ethanol and octanoic acid, to whip up ethyl octanoate. Concentrated acid, often sulfuric, acts as a catalyst, and the process demands heat and careful removal of water to push the reaction. Temperature control ensures the yield doesn’t drop. Industrial plants set up continuous distillation to separate the ester from leftover alcohol, water, and acid. Some producers capture “green” cred by working with bio-based feedstocks, meaning their octanoic acid often starts its life as part of a natural oil or fat. Innovations include solid acid catalysts and lower-energy approaches for companies chasing environmentally friendly production. After purification, storage in airtight drums prevents the loss of those precious aromatic notes.
Beyond straightforward esterification, ethyl octanoate shows up in research as a starting point for other chemical tweaks. Reductive hydrogenation turns esters like this into their respective alcohols. Some clever chemists deploy transesterification to swap out the ethanol piece, generating related esters for custom aromas. Controlled hydrolysis, often under alkaline conditions, breaks the compound down into ethanol and octanoic acid. In fragrance research, tweaking the chain length or swapping the alcohol builds analogs with subtle shifts—sometimes cranking up the fruit, sometimes bringing in coconut or floral shades. These modification strategies matter for companies targeting regional preferences or cost-effective alternatives to rare fruit extracts.
Reading product labels or research papers can sometimes seem like a scavenger hunt. Ethyl octanoate goes by names like ethyl caprylate, octanoic acid ethyl ester, or even fruity ethers in older texts. Flavor houses slap on trade names tailored to their blend lineups. The wine science crowd often sticks with “ethyl caprylate.” No matter the alias, the structure stays the same—a testament to the molecule’s staying power across industries and naming conventions. Shoppers should double-check labels and CAS numbers (123-66-0) to avoid mix-ups, especially when juggling food, pharmaceutical, and industrial-grade materials in the same facility.
Working with ethyl octanoate is usually safe for trained hands, but complacency breeds trouble. Its low flashpoint and volatility require proper ventilation and spark-free equipment. Inhalation won’t knock out a lab tech, but overexposure can cause throat and eye irritation. Skin contact rarely leads to serious complaints, though gloves and goggles remain non-negotiables in any properly run lab or processing area. Spill management calls for absorbent material and quick cleanup to keep walkways from turning hazardous. Companies look to OSHA, REACH, and the Globally Harmonized System (GHS) for step-by-step procedures. Emergency plans, safety data sheets, and regular training sessions keep the compound an asset rather than a liability.
Ethyl octanoate leaves a long trail across industries, but nowhere more than food and beverage. Winemakers chase it for enhancing tropical fruit profiles, boosting the complexity of chardonnay and sparkling wines. It finds its way into fruit candies, yogurts, and even non-dairy milks—all places where a single drop can shift an entire batch’s flavor character. Perfumers blend it with other esters and aldehydes for summer sprays and bath products. Animal feed producers sprinkle trace amounts into blends to mask off-notes and increase palatability. Occasionally, pharmaceutical formulations make use of it as a masking agent when tricky active compounds threaten to spoil a product’s taste. Each use counts on its longevity, stability, and ability to integrate with a product’s overall sensory experience.
University labs and corporate R&D teams continue to dig into ethyl octanoate’s characteristics. Analytical chemists refine detection methods, using headspace GC-FID or GC-MS, to trace even faint concentrations in grape must and finished wines. Sensory scientists drill down to threshold levels, pinning down how sensitive different consumers are to its aroma. New fermentation strategies let winemakers or brewers dial up or dial down production by selecting yeast strains or adjusting nutrient profiles. Biotechnology companies hunt for enzyme reactors that work under mild conditions, creating greener routes without sacrificing yield. Some groups push for computational studies, mapping receptors and predicting how tiny tweaks at the molecular level could reshape aroma profiles for niche consumer segments. R&D doesn’t stand still—market feedback, changing regulations, and cost pressures all keep the innovation wheel spinning.
Most safety reviews of ethyl octanoate feed into its “generally recognized as safe” status at the concentration levels used in food. Animal studies back up low acute toxicity and minimal risk from dietary exposures, although chronic or high-dose exposures in lab animals get more scrutiny for any subtle effects on the liver or nervous system. Regulators push for clear evidence that the ingredient does not build up in tissues or interact with medications. Studies funded by industry and independent governments keep a watchful eye on metabolites, breakdown routes, and possible interactions with sensitive groups like infants or those with liver disease. Outlier reports sometimes flag metabolites that could cause irritation, but nothing points toward major public health risks under normal usage guidelines. Staying current means reading the annual reviews and watching for any warning signals from international food safety agencies.
As the clean label movement drives demand for natural and sustainably sourced flavors, ethyl octanoate’s story continues to evolve. Producers ramp up investments in fermentation-based synthesis and digital tracking for each batch’s journey from raw material to end-user. Emerging markets in Asia and South America bring new culinary trends, drawing on ethyl octanoate’s ability to lift tropical and stone fruit profiles in both foods and drinks. Advances in green chemistry promise even lower-waste processes and better carbon footprints. Custom aroma tailoring for meat and dairy alternatives opens up another chapter, drawing on consumer preference studies and rapid prototyping. Regulatory agencies keep raising the bar, so transparency and documentation will only grow in importance. Ethyl octanoate stands as both a proven player and a testbed for next-generation food, fragrance, and chemical innovation.
Ethyl octanoate doesn’t show up splashed across product labels or catch your eye in the supermarket. Still, pour a glass of white wine, bite into a ripe pineapple, or open a bottle of fruit-flavored soda, and you’ve likely crossed paths with it. For years, I have taken a simple pleasure in uncovering the small details that shape big experiences—food chemistry sits among my favorites. Ethyl octanoate stands out because it shapes the way things taste and smell, without calling attention to itself.
Companies that make soft drinks, candy, and baked treats often chase one goal: crafting a flavor so close to the real fruit, you couldn’t tell it from nature. Here’s where ethyl octanoate earns its stripes. This compound, with its sweet, slightly fatty, and fruity scent, acts as a cornerstone in constructing flavors like pear, pineapple, and apricot. In my time talking with flavorists, I’ve found they lean on such molecules to fill in the gaps left by natural fruit extracts, which can shift in taste, expense, and long-term supply. Real fruit can be inconsistent from crop to crop, but ethyl octanoate delivers steady character and keeps the recipe predictable year-round.
Wine owes much of its aroma to compounds like ethyl octanoate. Winemakers watch its levels develop as yeast ferments grape juice, coaxing those signature tropical and floral notes that tip a bottle from forgettable to memorable. Ask sommeliers which aromas catch their attention, and this is one they’ll mention in a tasting note about young white wines. A sprinkle too much, and the result feels artificial. The right amount, and a chardonnay suddenly blooms with the scent of ripe pears.
Open any cosmetic or personal care aisle, and ethyl octanoate is quietly working behind the scenes. Perfume makers count on molecules that bring both depth and brightness, and this compound’s fruity profile fits perfectly in summery or gourmand scents. The formula may also turn up in room sprays and air fresheners, nudging the room toward the inviting embrace of citrus or tropical fruit. I’ve found even household cleaners use it. That fresh, sweet smell paired with the promise of cleanliness is often the work of these hidden helpers from the world of flavor chemistry.
In the business of adding flavor, keeping things safe and trusted sits right at the center. Authorities like the U.S. Food and Drug Administration—known for strict standards—classified ethyl octanoate as generally recognized as safe (GRAS) for use in foods. Long-term studies back this up, with no red flags at standard usage levels. Still, the wider use of synthetic additives continues to spark debate. Some people want a return to food made from plants in the garden, not a laboratory. Catering to these views calls not for sweeping rejection, but for openness—clear labels and making scientific safety checks public. Building understanding through conversation helps rebuild trust, which has lagged as the ingredient list on packaged foods has grown longer.
The modern food landscape revolves around taste, shelf life, and cost. Compounds like ethyl octanoate help companies meet demand without overusing limited natural resources. To win consumers' confidence, food makers can share clear stories about why such ingredients appear in their products, detailing how safety gets checked and how tiny amounts add up to big gains in enjoyment. Reimagining labels so people know what’s in their food, combined with strong oversight from safety agencies, can bring a new era where trust and taste go hand in hand.
Grocery stores and restaurants offer foods bursting with flavors, sometimes listing unfamiliar ingredients. Ethyl octanoate shows up in fruit-flavored products, baked goods, soft drinks, liqueurs, and sometimes perfumes. The label might not explain much about this chemical, but in the food world, it acts as a key aroma compound. It smells like pineapple or pear, and delivers a sweet, rich flavor in tiny amounts.
Scientists have studied ethyl octanoate for decades. This ester appears naturally in many foods, from apples to brandy. Flavor chemists in the industry even extract it from fermentation, where yeast breaks down sugars to give wine and beer their signature notes. The FDA calls it “generally recognized as safe” (GRAS) for use in food. The Joint FAO/WHO Expert Committee on Food Additives reviewed it too, giving it a green light at the low concentrations added for flavor.
Growing up in California’s Central Valley, surrounded by orchards and vineyards, I developed a respect for the subtle art of flavor. Ethyl octanoate arises in ripe fruit, showing nature’s own way of crafting these tastes. At family gatherings and local fairs, we sipped cider, ate homemade pies brimming with peaches and grapes, and never thought twice about the molecules that brought the flavors together. Over years of snacking and sampling, I never encountered issues linked to this compound, nor did anyone in my community.
Chemists measure the amounts used for flavoring in parts per million, so a pie or soda contains a fraction of a milligram per serving. In animal studies, large doses—over a thousand milligrams per kilogram of body weight—produced no significant problems. Every substance, even water, can pose danger at absurd levels, but nobody eats enough ethyl octanoate for this to matter.
Many people worry about artificial ingredients in food. It’s smart to pay attention to what gets added to the food supply, and every so often a new study makes headlines. For ethyl octanoate, the data keep coming back with the same message: the ingredient doesn’t pose health risks at the levels used for flavor. No strong evidence connects it to any chronic illnesses, allergies, or strange side effects.
Transparency in labeling and stricter oversight help keep the food supply safe. Consumer advocacy groups encourage manufacturers to share information about all additives, not just the ones that might sound scary. Scientists and regulatory agencies keep reviewing the literature, updating guidance when new findings pop up. For people who like to double-check the science, resources from the FDA and European Food Safety Authority provide plain-language answers and technical reports.
If avoidance feels best for your peace of mind, sticking with whole fruits and homemade drinks stays an option. For most folks, enjoying treats and flavors with ethyl octanoate in moderation looks safe. Watching trends in food science and staying informed about new research helps keep everyone healthy and reassured.
A lot of flavor gets more credit than it deserves for coming from fruit. Take a good whiff of a ripe pear or pineapple, and you’re meeting ethyl octanoate. For years working with small wineries, I noticed how much attention people gave to fermentation protocols and aging in barrels. All the while, the unsung heroes—esters like ethyl octanoate—carried the signature aromas of fruit. This chemical packs a punch with tropical and juicy notes that set apart wines and juices with characteristic freshness.
For those who’ve walked into a fragrance shop, the burst of fruit isn’t coming from a basket behind the counter. Ethyl octanoate rounds out perfumes, finishing formulas and layering them with a pleasant, sweet top note. Top brands rely on its effect to turn an ordinary scent into something fresh and inviting. Without a chemical like this, fragrance work becomes a lot more muddled—lacking those crisp, vivid layers that attract loyal customers.
Ethyl octanoate finds its way into soft drinks, candies, and dairy products to create the taste we expect. Soda companies pay careful attention to compounds like this because they can make or break a product launch. My time in a food lab taught me that a lot of what seems “real” in taste tests is the handiwork of a molecule like this. Regulatory groups, especially in places like the United States and Europe, review its use and set limits. Data from food safety authorities—such as the Flavor and Extract Manufacturers Association—support its use at low concentrations, and industry keeps researching to cover all health concerns.
Beyond the fancy side of food and luxury, ethyl octanoate shows up in less obvious corners. Cleaning products get a scent boost from it, helping mask harsh smells and delivering a sense of cleanliness. In lubricants and plastics, it’s used to give flexibility to materials and even reduce static. These applications get far less attention, but I’ve seen manufacturers turn to this chemical as a simple fix for stubborn odor or performance problems. It’s not dramatic, but it matters plenty to the folks working the late shift at a factory.
Whenever a chemical gets this much play across so many fields, the question of safety lingers. Research points to low toxicity and quick breakdown in the environment at the levels it’s used. Yet history has shown surprises, so it’s smart to support more transparent, independent testing. Companies and regulators keep records accessible, but the field could use more public reporting.
With so much built on the back of one small molecule, it pays to stay alert. Supply chains, safety testing, even producer training—all parts of the process could use more investment. Brands that communicate openly about sourcing and safety earn consumer trust. And for new chemists stepping into the field, the lesson is clear: even the smallest player in a product can carry a big responsibility.
Anyone who has ever worked with fruits, wine, or flavors probably crossed paths with something called Ethyl Octanoate. I remember nosing a glass of young white wine and instantly feeling like I’d just walked past a summer fruit stand. This stuff is responsible for an aroma that jumps out of the glass—a punch of fresh, sweet fruit, especially pear and pineapple.
If you slice open a juicy Bartlett pear and catch that rush of fragrant air, what you’re getting is very close. Ethyl Octanoate packs that same vibrancy into flavors and aromas. Sometimes it even pulls in softer melon or mango characters, depending on the concentration and the company it keeps in a mix.
Getting a sense for this molecule in your mouth is even more straightforward. There’s an almost candied, ripe quality to it—fresh fruit salad meets hard candy. I’ve done side-by-side sniffs of pure esters, and this one is hard to forget: a tropical blast with no sharp edge. Cheesemakers get it in certain aged cheeses, though, in wine and spirits, the impression is pure sunshine in a glass.
Winemakers chase it especially for that reason. California Sauvignon Blanc, for example, sometimes pushes forward a bold fruit punch—Ethyl Octanoate helps give those wines an irresistible, energetic candy edge. In apple brandies, the ester rounds out crisp, tart flavors and leans in with a supple, banana-toned layer.
I’ve seen food labs tweak concentrations down to the microgram, since just a pinch too much sends the flavor profile into awkward, almost chemical candy territory. Its intense fruitiness doesn’t blend well with everything. Perfume and food scientists always keep their hand on the dial, because small shifts can mean the difference between “freshly sliced” and “artificial.”
In my kitchen testing, adding a drop to a neutral spirit made the difference between nondescript alcohol and a vibrant homemade liqueur reminiscent of pear schnapps. Even in soft drinks and candy, it serves as a backbone—an invisible helper, making commercial fruit flavors pop, stand out, and seem more real.
Balancing Ethyl Octanoate isn’t just about pushing for maximum fruitiness. Fun fact: wine experts use “threshold” testing, having tasters sample liquids with ascending amounts until most agree on the moment the fruit character jumps out. High-quality control in food or beverage plants means using precise measurement, then sensory evaluation. This reduces the chance of overdoing the intensity and skews flavors toward authenticity rather than a cheap “fruit-flavored” impression.
For everyday consumers, recognizing this molecule is a way to read wine labels or taste descriptions more critically. If something promises “tropical” or “juicy pear,” odds are Ethyl Octanoate had a hand. Next time you open a well-made fruit juice, candy, or bottle of white wine, inhale deeply. That rush of summer orchard aroma signals all the skill and chemistry that goes into making flavors feel real.
Science supports the power—and risk—of using Ethyl Octanoate. Studies show humans detect it at extremely low concentrations, sometimes less than one part per million. Producers looking to make more authentic and nuanced flavors build sophisticated sensory panels—for wine, soft drinks, and even bread doughs—that focus on real-world testing instead of just lab data. This approach helps avoid the synthetic “overdone” effect that sometimes knocks processed foods off balance.
Guided by authentic aroma and taste experience, the best producers keep flavor creation rooted in what real fruit lovers crave: honesty, freshness, and restraint. In the end, nailing the right level of Ethyl Octanoate brings out the best in beverages, baked goods, and candies—without drowning out the complexity that nature provides.
Ethyl octanoate stands out in the world of food and fragrance because of its strong fruity aroma, often described as similar to pineapple or apricot. For most people, that scent is a pleasant little surprise in a glass of wine or orange juice. What’s interesting is that ethyl octanoate shows up both in living things and in labs.
Nature has a way of crafting molecules that seem engineered for delight. Ethyl octanoate forms naturally in ripe fruits and is especially noticeable in various wines. During fermentation, yeasts work on sugars and create a range of byproducts, and this ester comes out as a natural result of their digestion. Its impact on flavor profiles in certain wines, especially in whites and sparkling varieties, has been noted by winemakers for years.
If you’ve ever lingered over a glass of Muscat or even sniffed at apple cider, chances are this ester hit your nose. Fruits like apricots and even some aged cheeses contain trace amounts as well, proving that it’s not just a product of industrial chemistry.
Chemically speaking, ethyl octanoate is an ester created from octanoic acid and ethanol. Nature produces both ingredients; bacteria and fungi churn out fatty acids, while ethanol surfaces during fermentation. The body itself produces small amounts, and researchers have even found ethyl octanoate in human breast milk.
Lab tests have shown that its concentrations in foods and beverages aren’t just trivial. A study in the “Journal of Agricultural and Food Chemistry” highlighted its role in tropical aromas in New Zealand Sauvignon Blanc. Its presence isn’t limited to just rare or obscure places—it's part of what shapes everyday taste and scent experiences.
Even though nature takes care of ethyl octanoate in fruits and barrels, the food and fragrance industry needs large amounts. Every piece of mass-produced candy, soda, or perfume promises the same flavor or scent from batch to batch. Relying on fruit or fermentation alone would leave companies at the mercy of the seasons. Sugar levels in grapes fluctuate based on rain, and some years produce more ester than others.
To meet the need, companies turn to chemistry. Producing esters in the lab isn’t some nefarious plot but a practical decision. Reacting ethanol with octanoic acid makes ethyl octanoate in larger, more predictable amounts. This synthesized version is chemically identical to the one found in apricots or a Chardonnay.
For folks trying to eat fewer “artificial” ingredients, the label game is tricky. Just because something comes from a lab doesn’t make it unsafe. The real thing and the synthetic version act the same way in our bodies. Concerns should focus on the entire product and its overall nutrition, rather than the source of this ester alone.
Regulation by agencies like the US FDA and EFSA helps keep both natural and synthetic additives within safe limits. Food science keeps evolving, guided by both research and tradition. At the end of the day, whether from a grape or a beaker, ethyl octanoate brings a little happiness to a lot of kitchens.
| Names | |
| Preferred IUPAC name | Ethyl octanoate |
| Other names |
Ethyl caprylate Octanoic acid ethyl ester |
| Pronunciation | /ˈiːθɪl ɒkˈteɪ.ə.neɪt/ |
| Identifiers | |
| CAS Number | 106-32-1 |
| Beilstein Reference | Beilstein Reference: 1721359 |
| ChEBI | CHEBI:51112 |
| ChEMBL | CHEMBL3184717 |
| ChemSpider | 8165 |
| DrugBank | DB14057 |
| ECHA InfoCard | 100.110.823 |
| EC Number | 205-434-3 |
| Gmelin Reference | Gmelin Reference: **11113** |
| KEGG | C10447 |
| MeSH | D016621 |
| PubChem CID | 7792 |
| RTECS number | RA0725000 |
| UNII | 495PR8Y1Z9 |
| UN number | UN1197 |
| Properties | |
| Chemical formula | C10H20O2 |
| Molar mass | 172.26 g/mol |
| Appearance | Colorless liquid |
| Odor | fruity |
| Density | 0.86 g/cm3 |
| Solubility in water | 3.4 mg/L (at 25 °C) |
| log P | 3.49 |
| Vapor pressure | 0.077 mmHg (25°C) |
| Acidity (pKa) | 14.06 |
| Basicity (pKb) | pKb ≈ 15.86 |
| Magnetic susceptibility (χ) | -68.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4150 |
| Viscosity | 0.751 cP (25°C) |
| Dipole moment | 1.78 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 527.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -413.4 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -5222.7 kJ/mol |
| Pharmacology | |
| ATC code | '' |
| Hazards | |
| GHS labelling | GHS07, Exclamation mark |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P301+P310, P303+P361+P353, P304+P340, P305+P351+P338, P312, P321, P330, P337+P313, P362, P370+P378, P403+P235, P501 |
| Flash point | Flash point: 107°C (225°F) |
| Autoignition temperature | 225 °C |
| Explosive limits | Explosive limits: 0.7–7% |
| Lethal dose or concentration | LD50 (oral, rat): 12,800 mg/kg |
| LD50 (median dose) | > 5.0 g/kg (rat, oral) |
| NIOSH | KK8225000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 5 mg/L |
| Related compounds | |
| Related compounds |
Octanoic acid Octanoyl chloride Methyl octanoate Butyl octanoate Ethyl acetate |