Ethylhexyl lactate has roots in the broader field of lactic acid ester chemistry, which picked up real steam in the 20th century as industrial needs shifted away from heavier, more problematic solvents and toward safer, more biodegradable ingredients. In the early days, lactic acid simply served the food and beverage or pharmaceutical markets. Chemists noticed that its esters — particularly those with branched alcohols — could open up broader applications, offering lower volatility and improved solubility profiles. Companies scaling up green chemistry in the 1970s and 1980s recognized ethylhexyl lactate as an alternative to traditional, toxic solvents and as a sustainable option. Slowly, it made inroads into cosmetics, coatings, and cleaning agents. My early career in chemical manufacturing saw long meetings debating the merits of greener profiles like these, but the shift carried real weight once regulations started moving toward safer, less persistent molecules.
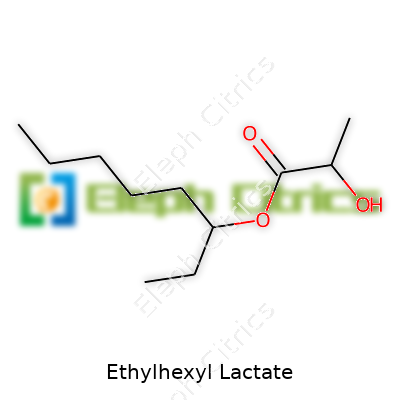
Ethylhexyl lactate, often labeled as 2-ethylhexyl lactate, falls under ester solvents derived from the reaction between lactic acid and 2-ethylhexanol. It presents as a clear, colorless liquid with a faint, almost fruity aroma. Unlike some harsh solvents, it features relatively low toxicity and excellent skin compatibility, which explains its popularity in personal care. Manufacturers package it largely in metal drums or HDPE containers, with purity grades tailored for industrial use or sensitive cosmetics and pharmaceutical products. Chemical suppliers compete on purity, odor, and trace impurity content, responding to company batch requirements. Out on the shop floor, handling ethylhexyl lactate feels a lot like working with light oils — not especially hazardous so long as you follow guidelines — yet better for health than heavier petrochemical solvents.
This liquid sports a boiling point near 250°C and a relatively low freezing point, remaining workable in diverse temperatures. A modest specific gravity hovers around 0.96. Miscibility with many common oils and organic solvents has boosted its reputation as a useful blending agent, while partial miscibility with water means engineers sometimes use it in emulsions or as a co-solvent in aqueous formulations. Its pleasant, mild odor and low volatility minimize workplace exposure and reduce the chance of strong, unpleasant fumes in consumer-facing products. The ester group confers moderate polarity, balancing solubility for both hydrophobic and hydrophilic compounds. Chemical stability over a reasonable pH range means less tendency to break down under tough storage or application conditions.
Suppliers provide technical data sheets that detail purity (usually ≥98%), water content, acid value, refractive index, and color as measured by the APHA scale. Material safety data sheets spell out hazards — low but not negligible: eye irritation can occur, and prolonged dermal exposure is best avoided. In my time updating procurement systems, I recall suppliers following GHS labeling strictly, flagging the need for standard PPE. Regulatory agencies insist on exact labeling: CAS number 19761-38-3, batch information, manufacturing date, expiration date, and traceability through supply chains. Price can swing based on crude oil (affecting 2-ethylhexanol) and the lactic acid feedstock, especially when crop failures reduce fermentation yields.
Manufacturers produce ethylhexyl lactate through direct esterification of lactic acid with 2-ethylhexanol. Acid catalysis — often sulfuric acid — produces high conversion rates in batch reactors at moderate temperatures, followed by distillation and vacuum stripping to purify the product and drive the reaction to completion. Some teams have experimented with solid acid catalysts for easier purification. My own experience working on green process development showed that water removal through azeotropic distillation sharply increased yield; anyone running this process learns the value of a steady hand controlling reflux ratios and distillation cuts.
While stable under ordinary conditions, ethylhexyl lactate can hydrolyze back to lactic acid and 2-ethylhexanol in the presence of strong acids or bases and water. In practical applications, this rarely crops up, but in high pH cleaners or alkaline formulations, you see more concern for shelf-life. Its ester group means that mild oxidation can break the molecule down; keeping containers sealed and stored under nitrogen sometimes pops up as a precaution in high-precision or high-purity sectors. Chemists occasionally transform it further, using the lactate as a building block for specialty surfactant production or modifying the ester group to fine-tune solubility. On rare occasions, enzymatic hydrolysis gets tested in biocatalytic clean-up processes.
You spot ethylhexyl lactate on the market under a raft of synonyms, including 2-ethylhexyl 2-hydroxypropanoate, octyl lactate, or simply as a branded ingredient in specialty formulations. In personal care, some companies market it under trade names that allude to smoothness or emollience. Lab catalogs may still use older nomenclature or highlight specific diastereomer ratios. In practical factory terms, there’s little confusion as labeling standards have tightened up, but any incoming chemical should always match CAS numbers to avoid supply hiccups.
Ethylhexyl lactate scores relatively well for safety, but like every industrial chemical, it has its risks. Eye and skin irritation can result from splash exposure, and ingestion leads to stomach distress, so best practice calls for gloves, goggles, and routine ventilation during handling. Safety audits in facilities storing thousands of liters flag spill containment and mandatory eyewash stations near transfer points. It easily meets REACH registrations in the EU and falls under TSCA inventory in the US, making compliance simpler on both sides of the Atlantic. Workers receive standard hazard training, with extra focus on preventing vapor buildup, especially in enclosed mixing rooms. Fire risk remains low given its high flashpoint, easing insurance assessments. Still, chemical hygiene protocols remain a fixed requirement, not just for regulatory box-ticking, but to maintain a culture of safety.
Ethylhexyl lactate shows up in a surprising number of products. Personal care manufacturers use it for its mildness and its ability to dissolve sunscreen actives, dyes, and fragrances. In my sampling projects, lotions and liquid foundations always performed better — in terms of feel and blend — when this ester replaced more volatile alternatives. The paint and coatings sector values its solvency for resins and pigments; the lower evaporation rate gives better leveling and film formation. In cleaning, floor polishes and hard surface detergents employ it for degreasing, with far less irritation than glycol ethers or other strong solvents. Pharmaceutical teams occasionally adopt it in topical drug delivery systems, since it doesn’t easily trigger allergic responses and helps disperse actives. The rise of “clean” labels and consumer green shifts means I’ve seen growing demand for this molecule in everything from baby wipes to eco-friendly paint strippers.
Recent years have brought advances in both synthesis and application. Academic researchers work on process intensification, including continuous-flow reactors for faster, less resource-intensive production. Analytical chemists dig into impurity profiles and trace byproduct removal, critical for cosmetics and food-contact markets. Formulation scientists keep testing its ability to enhance solubility of actives that barely dissolve in water-based systems. At industry trade shows, I see R&D leaders pushing for improvement in odor control and higher purity, driven by the expanding Asian and European regulatory landscape. Multiple patent filings focus on blends with natural surfactants or greener preservatives, where the lactate group stabilizes sensitive actives or shifts pH for extended shelf-life. Industry insiders discuss sustainable sourcing for lactic acid inputs, such as non-corn carbohydrates or cellulosic biomass, aiming to close production gaps during bad harvest years.
Toxicology studies over the past decade tend to highlight low acute toxicity, limited absorption through intact skin, and a lack of systemic effects after topical exposure, which have encouraged personal care brands to switch reformulations. Long-term feeding studies on rats and mice, required for regulatory approval, report high no-effect levels, while human patch tests seldom trigger severe reactions. Researchers remain cautious due to isolated reports of eye irritation and mild sensitization, especially among workers with repeated, prolonged contact. For manufacturers, technical measures such as closed pumping systems, improved ventilation, and educational safety briefings continue to minimize workplace issues. A notable point: environmental toxicology work shows ethylhexyl lactate rapidly biodegrades in soil and water, cutting the risk of groundwater buildup. These results set the molecule apart from legacy solvents with known bioaccumulation risks.
Prospects look strong as consumer and industry pressure for safer, renewable ingredients takes off. Global regulations aim for lower VOCs, non-persistent organics, and higher workplace standards, all of which play to ethylhexyl lactate’s strengths. Its core synthesis links easily to fermentation-derived lactic acid, letting companies tout both green chemistry credentials and circular feedstock chains. Innovations in continuous-flow synthesis, biocatalysis, and integrated production facilities hold promise for lower costs and better traceability. The molecule’s mildness suits a generation of personal care and cleaning products fighting for dermatologist seals and “free from” labels. Research teams continue pressing for greater purity, broader compatibility with biodegradable polymers, and expanded use in pharmaceutical carriers. The main challenge remains keeping raw lactic acid streams steady amid crop fluctuations and developing supply chains that can handle the move from niche to mainstream without choking on logistics costs.
Ethylhexyl lactate often pops up in ingredient lists for personal care products like sunscreens, cleansers, and makeup removers. I spent years flipping bottles in store aisles, wondering how a single compound gets around so much in the beauty world. Ethylhexyl lactate works as a skin conditioning agent and a solvent. It boosts texture and gives products that easy-to-spread feel, creating lotions that glide smoothly and don’t sit heavy on the face or arms.
Manufacturers choose ethylhexyl lactate largely because it helps cut greasiness. It breaks down heavy creams, so formulas don’t go on sticky or oily. This lightweight quality appeals to big companies chasing that “barely-there” sensation customers want in daily moisturizers or facial serums.
Ethylhexyl lactate also plays a big part in sun protection products. It helps disperse and stabilize UV filters, so sunscreen shields skin better against sun damage. This matters more than ever, given reports about rising skin cancer rates and consumers seeking broad-spectrum coverage. A sunscreen that leaves a white cast or pills under makeup rarely earns repeat customers. By fostering smooth application and proper absorption, ethylhexyl lactate supports the performance of SPF products in the real world.
I’ve noticed ethylhexyl lactate in micellar waters and facial wipes, too. It loosens stubborn makeup and sunscreen, so you don’t have to rub skin raw just to get clean. The real-world win comes at the end of a long day, seeing eyeliner slide off in seconds without burning or stinging.
The flexibility of ethylhexyl lactate tempts skincare chemists regularly. It's sourced from lactic acid and 2-ethylhexanol, which both come from wider industrial processes already proven safe in regulated quantities. Cosmetic researchers point to its biodegradability, which lines up with more brands trying to cut their eco-footprint. Its compatibility with natural, synthetic, fraganced, and fragrance-free formulations gives brands the freedom to cater to allergy-prone or sensitive skin customers.
Regulators such as the European Chemicals Agency and the US Food & Drug Administration permit its use in cosmetics, setting specific concentration limits. Most products with ethylhexyl lactate keep it well below these cut-offs. Reports of skin irritation are rare, though some people with extremely reactive skin prefer to spot-test beforehand.
Even as ethylhexyl lactate pulls its weight in personal care, stories about increased skin allergies and sensitivities grab attention. People raising concerns over “chemical names” in formulations show up more often in online skincare forums. Transparent labeling and education, using plain language to describe why each ingredient gets picked, can rebuild trust between companies and buyers.
Brands seeking a bigger share of the clean beauty market test lower concentrations or blend ethylhexyl lactate with botanicals to soothe skin. The real challenge lies in keeping products stable, performing well, and feeling good on skin without cutting corners. Industry experts highlight ongoing research into plant-derived alternatives, all chasing that same smooth, lightweight finish.
Ethylhexyl lactate doesn’t exist for show on an ingredient label. It solves real-world problems: better feeling creams, sunscreens that spread evenly, makeup that’s easy to wipe away. As consumer expectations shift toward safety and sustainability, chemists dig deeper into sourcing and alternatives but often circle back to proven helpers like this one. The dialogue between transparency, science, and customer comfort keeps shaping what shows up next on those bottles.
Many people scan ingredient labels, hoping to avoid skin problems or allergic reactions. Ethylhexyl lactate pops up a lot in cosmetics, sunscreens, and personal care products. Chemists use it to help mix formulas smoothly or boost the way a product feels on skin. The real question lingers: does it belong in something you put on your face daily?
Dermatologists and toxicology experts dig into ingredients from a scientific angle. Research published by trusted groups, such as the Cosmetic Ingredient Review panel, points out that ethylhexyl lactate brings low skin irritation risk, especially at the levels seen in consumer skincare. Even in spot tests, people rarely experience itchiness or redness from it. I have sensitive skin myself, and I lean toward products that keep the risk of irritation down. Many dermatologists I’ve talked to share this approach.
The U.S. Food and Drug Administration keeps an eye on cosmetic ingredient lists, though it doesn’t require pre-approval for most components in lotions or creams. Larger companies run patch tests and report negative results quickly. If an ingredient keeps showing a safe profile, companies stick with it. Ethylhexyl lactate falls into that bucket, according to available data.
With so much concern over microplastics and chemical buildup in nature, the source of each ingredient starts to matter. Ethylhexyl lactate traces back to natural lactic acid and fats. It doesn’t linger in the environment like some silicones or plastics. The European Chemicals Agency reports it breaks down fairly quickly in water and soil.
I try to use eco-responsible products at home, especially for my family’s sake. Plenty of brands aiming for a low environmental footprint include ethylhexyl lactate for these reasons. I’ve noticed more eco-certified products listing it on the label.
Even plenty of safe ingredients might trigger trouble in rare cases. If you have a known allergy to lactic acid or other esters, any breakouts or redness could signal a problem. Years ago, a friend of mine found herself itchy after sampling a new moisturizer with ethylhexyl lactate high on the ingredient list. After stopping use, her skin settled down, though her reaction was unusual.
Consumer data from databases like EWG's Skin Deep show that most folks use this ingredient without a hitch. Still, new users should try spot testing, especially if they deal with eczema or strong reactions. Health Canada and the EU Cosmetic Regulation both greenlight ethylhexyl lactate for rinse-off and leave-on products, further supporting its safety.
For people who feel safest using the bare minimum, fragrance-free and simple formulas often cut out less common esters. Shopping small-batch or hypoallergenic options works well for my own skin, and many dermatologists recommend asking for samples before buying in bulk. The beauty industry has shifted toward transparency—ingredient lists get longer but also clearer.
Ethylhexyl lactate doesn’t show up as a common skin allergy culprit. Most evidence points to its place as a gentle helper in formulas, backed by scientific studies and industry oversight. Still, if something doesn’t work for you, listen to your skin and talk to a professional. That level of attention builds long-term trust—not just with a product, but with your own skin health.
Reading the label on a bottle of sunscreen or moisturizer, you might notice ethylhexyl lactate peeking out from the long list of ingredients. The question comes up: is this stuff natural or is it cooked up in a lab somewhere? That isn’t just trivia for ingredient nerds. The answer points back to how we handle safety, sustainability, and even marketing in the beauty and personal care world.
Ethylhexyl lactate is an ester built from lactic acid and 2-ethylhexanol. Lactic acid has a good story—it’s already found in nature, hanging around in sour milk and created by your muscles when you work out hard. Ethylhexanol, on the other hand, doesn’t show up in fruits or vegetables. Industry usually pulls it out from petrochemicals. So, when ethylhexyl lactate arrives on shelves, it comes from a mix: the lactic side often has a natural origin (think corn or beets being fermented by friendly bacteria), but the ethylhexanol side leans toward synthetic chemistry.
Walking down a grocery aisle, anything labeled natural seems like it should be simple and pure. I’ve learned those terms often blur at the edges. With ethylhexyl lactate, calling it natural or synthetic isn’t as black and white as many expect. Some brands try sourcing both building blocks from bio-based feedstocks, but most of what goes into skin care or cosmetics involves a synthetic part. Companies love the flexibility of synthesis—it slashes costs and helps them control quality.
Truth is, the “naturalness” of an ingredient doesn’t make it safe by default. Plenty of natural substances can irritate skin or trigger allergies; synthetic chemicals can be harmless or even safer, depending on standards and controls. Research on ethylhexyl lactate, backed by Cosmetic Ingredient Review (CIR) findings, shows it generally causes little irritation or sensitization at levels used in products. Regulatory bodies like the FDA and the EU keep tabs on usage and safety, so it’s not a wild west scenario.
Consumers wanting sustainable options have pushed companies to rethink sourcing. There’s some movement toward making both lactic acid and ethylhexanol from renewable crops. The reality? Most ethylhexyl lactate today traces back to fossil fuels for at least half the process. That’s where the pressure lands: companies face tough choices between price, reliability, and environmental impact. The technology for fully bio-based versions exists, but higher costs and supply obstacles slow things down.
In my experience, people care less about an ingredient’s chemistry degree and more about what it means for their health and for the planet. Brands earn real trust when they skip the vague “natural” claims and give people the facts. A simple statement on the label about where key ingredients come from and how they’re made gives shoppers the power to decide what matters to them. If the personal care giants want to set themselves apart, honest sourcing details and third-party certifications speak louder than green-sounding taglines ever could.
Ethylhexyl lactate shows up on the ingredients list of plenty of cosmetics and personal care items. Companies turn to it because it helps products glide on the skin, adds a silky feel, and works as a mild solvent. You’ll see it in sunscreens, lotions, and sometimes even facial cleansers. The ingredient comes from lactic acid and 2-ethylhexanol, so on the surface, it sounds simple and safe. But sometimes people want to know if their skin will react badly to it, and that's worth a closer look.
Complaints about stinging or redness from lotion aren’t rare. Friends have told me about certain face creams turning their skin blotchy, and they always wonder which ingredient to blame. Dermatology researchers have tested ethylhexyl lactate in lab and real-world settings. Studies published in journals like Contact Dermatitis reported patch testing, and the rates of allergic reactions from ethylhexyl lactate remain pretty low. That matches my own experience as someone who’s tried dozens of skincare products; I haven't had reactions to this chemical, and most friends don’t mention it either.
Yet, everyone’s skin differs. Dermatologists point out that some people have a higher chance of sensitivity—kids, older adults, anyone with eczema or a history of skin allergies. Those groups sometimes notice burning, tingling, or a rash after using something new. Just because an ingredient doesn’t bother the majority doesn’t mean it’s a perfect fit for all.
Much of the irritation linked with ethylhexyl lactate happens if someone uses a product with a high concentration, or if they already have damaged or delicate skin. The ingredient itself doesn’t belong to the group of high-alert allergens flagged by dermatologists, like fragrances or certain preservatives. Still, some folks with sensitive skin see a mild reaction—redness, dryness, maybe a little itch. Allergic reactions (true allergic contact dermatitis) pop up less often, but they’re not impossible. The American Contact Dermatitis Society doesn’t put ethylhexyl lactate on their most common allergen lists, but patch testing centers do include it because a small portion of people will react.
Takeaway from both scientists and personal stories: if your skin has a history of flaring up, read product labels and pick formulas for sensitive skin or labeled fragrance-free. I like to test a new face product on a small patch of skin, waiting 24 hours before using it everywhere. That’s a habit picked up after hearing one too many “rash after one use” stories from people I know.
If a reaction happens—a rash, swelling, or burning—wash your skin with gentle soap, skip the product, and if it sticks around, talk to a dermatologist. For manufacturers, using lower levels of ethylhexyl lactate and avoiding unnecessary additives can limit the chance of reactions. Dermatologists keep recommending patch tests in clinics for anyone struggling with recurring rashes on the face or body.
Ingredient transparency and personal awareness matter most. Journals, health organizations, and skin experts all suggest that allergic reactions from ethylhexyl lactate are rare but possible. Most people can use products with this ingredient without trouble, but the small number who react benefit from knowing about it and having choices in what they put on their skin. Paying more attention to our own skin, reading studies by trusted medical groups, and sharing experiences helps everyone make better choices for their unique needs.
Ethylhexyl lactate often ends up as a supporting player in creams, sunscreens, and lotions. Manufacturers appreciate how it thins heavy formulas and helps them spread smoothly. This ingredient, derived from lactic acid and a fatty alcohol called 2-ethylhexanol, lives in the emollient world. It keeps products silky and helps moisture stay where it belongs—on the surface of your skin.
People with sensitive skin watch ingredient lists like a hawk. One wrong move can mean redness, stinging, or mysterious bumps. I understand this painstaking dance with daily personal care—anything that burns or tingles never comes back into my bathroom cabinet. Navigating aisles packed with unpronounceable names, I look for gentle, proven ingredients. Fragrance-free, dye-free, and the shortest list possible tend to work best. Having watched friends and clients react to “gentle” products, real caution matters.
Research into ethylhexyl lactate doesn’t link it strongly with irritation or allergic reactions. Compared to stronger chemical exfoliants or common triggers like alcohol denat., this emollient sits low on the irritation scale. Cosmetic Ingredient Review (CIR) panel data, accessible since 2011, supports its use without major warnings for healthy adults. They report only rare cases of irritation, and those usually involve leave-on applications in higher-than-typical concentrations. Dermatologists occasionally flag it for allergy-prone folks if there’s a previous history of chemical sensitivity, but it lacks the notoriety of fragrances or preservatives such as parabens and formaldehyde releasers.
In my own experience, I’ve used lotions with ethylhexyl lactate across different skin types, including on clients known for highly reactive skin. Reactions felt rare and mild, far less dramatic than what I’ve witnessed from citrus oils or classic alcohols. Still, a reaction is never impossible for any ingredient. No one formula fits every person, especially when genetic and environmental factors play a part.
Patch testing stands out as the gold standard for anyone trying a new ingredient—especially if rashes and redness show up easily. Before slathering a new cream with ethylhexyl lactate across a cheek or forehead, place a bit on the inner wrist or side of the neck. Watch for any change over two days. Reading the full label also matters; ethylhexyl lactate might play it safe, but if it hangs out with harsher preservatives, the formula could set off trouble.
Sensitive skin thrives on simplicity. Choose products with short ingredient lists and skip anything promising overnight miracles. Moisturizers without fragrance, artificial color, or strong acids usually get along best with reactive complexions. Those already managing skin issues like eczema or rosacea benefit from dermatologist guidance before adding something new. Ethylhexyl lactate may have a safe track record, but each skin tells its own story.
Trust in skin signals comes before any marketing claim. If a gentle moisturizer with ethylhexyl lactate causes burning or itching, it won’t suit your skin, no matter the reputation or science. Safe choices weigh on real-life feedback and simple habits—patch test, monitor, and listen. With these steps, sensitive skin often finds its balance.
| Names | |
| Preferred IUPAC name | 2-ethylhexyl 2-hydroxypropanoate |
| Other names |
Lactic acid, 2-ethylhexyl ester 2-Ethylhexyl 2-hydroxypropanoate 2-Ethylhexyl lactate Octyl lactate |
| Pronunciation | /ˈiːθɪlˌhɛksɪl ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 626-68-6 |
| 3D model (JSmol) | `/d2- cccc(c)coC(=O)cC` |
| Beilstein Reference | 1721423 |
| ChEBI | CHEBI:86258 |
| ChEMBL | CHEMBL2035201 |
| ChemSpider | 150581 |
| DrugBank | DB11282 |
| ECHA InfoCard | 03b09b6b-9f8d-410a-913e-74d40fbdf6af |
| EC Number | 230-026-9 |
| Gmelin Reference | 530039 |
| KEGG | C14361 |
| MeSH | D02.455.326.271.465.432 |
| PubChem CID | 87751 |
| RTECS number | OG6300000 |
| UNII | 1F7KE1F2AT |
| UN number | UN1198 |
| CompTox Dashboard (EPA) | DTXSID3039247 |
| Properties | |
| Chemical formula | C11H22O3 |
| Molar mass | 202.29 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Faint odor |
| Density | 0.92 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 2.68 |
| Vapor pressure | 0.01 mmHg (20°C) |
| Acidity (pKa) | 16.03 |
| Basicity (pKb) | 15.1 |
| Magnetic susceptibility (χ) | -8.0×10⁻⁶ |
| Refractive index (nD) | 1.4300 |
| Viscosity | 15 mPa·s (25°C) |
| Dipole moment | 2.66 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 489.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -748.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3687.7 kJ/mol |
| Pharmacology | |
| ATC code | D11AX19 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P280, P305+P351+P338, P337+P313 |
| Flash point | > 110°C |
| Autoignition temperature | 215 °C |
| Lethal dose or concentration | LD50 (Rat, oral): > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 5,000 mg/kg |
| NIOSH | KHP499 |
| PEL (Permissible) | Not established |
| REL (Recommended) | <=1.0% |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Lactic acid Methyl lactate Ethyl lactate Propylene glycol Ethylhexanol Sodium lactate |