Ferrous lactate doesn’t draw as much attention as other ingredients in the nutrition field, but its story stretches back to the early explorations into dietary iron supplementation. You can trace its roots to a time when food fortification became a priority for public health officials. As soon as scientists figured out how to bind iron with lactic acid, they unlocked a form of iron that works well in both dietary and industrial uses, dodging some of the typical pitfalls of iron’s taste and stability in foods. The compound gained footing in Europe and the United States in the mid-20th century because food fortification programs wanted a solution that improved iron absorption without producing metallic flavors or unpredictable reactivity.
Ferrous lactate, a pale green crystalline powder, brings together iron and lactic acid in a form that blends into foods simply and keeps flavors from being distorted. Besides nutrition, its mild profile and reliable solubility mean manufacturers lean on it when formulating iron-rich health supplements. Some names you might run into on product labels include “Iron(II) lactate,” “E585,” and “lactic acid iron(II) salt.” Alongside its presence in foods, you’ll also notice it in pharmaceutical iron supplements and some personal care products. What stands out is its role not just as an essential nutrient source but as a stabilizer for other functional ingredients.
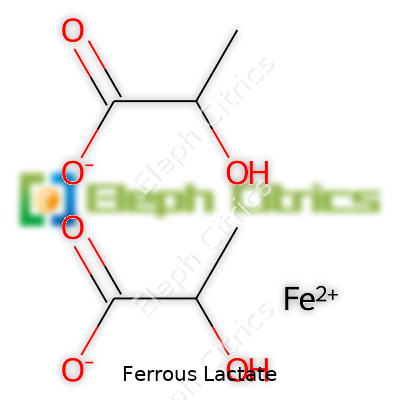
The physical structure of ferrous lactate—a slightly green, fine powder—serves more than just good looks for blending. It has a mild, nearly neutral scent and dissolves readily in water, which spells efficiency in beverages and liquid formulations. Chemically, this compound boasts stability across a range of pH levels, and the bond between iron and lactic acid resists breakdown during processing. The molecular formula is C6H10FeO6, and it balances an iron(II) ion with two lactate groups. Its solubility links directly to how well the body can absorb it, a major step above less soluble iron salts that encourage both nutrient loss and sense-numbing metallic flavors.
Listing ferrous lactate on a food or supplement label rarely brings clarity for most buyers, but behind those names lie standardized purity and dosage regulations. Various pharmacopeias set thresholds for iron content, moisture level, and contaminants. For example, nutrition agencies in both the US and Europe impose limits on lead, cadmium, and arsenic to protect users from exposure. The assays run by manufacturers look for iron concentrations between 18 and 22% by weight, and the particle size influences how efficiently it disperses. Health authorities ask for clear identifications, so it shows up under several recognized monikers—each batch sporting batch numbers, expiration dates, and origin details to trace the source if problems show up.
Preparation of ferrous lactate comes down to a chemical reaction between iron sources and lactic acid, typically at controlled temperatures and monitored pH. Most commercial producers start with high-purity iron filings or iron(II) salts and react them with lactic acid—sourced either from fermentation processes or chemical synthesis. Once the reaction forms the compound, it’s filtered to knock out insoluble impurities, then concentrated and dried to crushable powder or granules. Getting rid of excess reagents is key, as leftover acids or personal metals can tarnish the taste or safety. The right drying step keeps the iron in its usable bivalent state, avoiding oxidation that would turn it into a less-absorbable iron(III) variant.
Despite coming across as straightforward, ferrous lactate carries a complex set of reactions both in and out of the human body. Exposed to oxygen, it can oxidize to ferric lactate, changing color and nutritional punch. Some advanced processes tweak the lactic acid’s source (for instance, using specific bacterial fermentation) to refine purity or tweak binding characteristics. Research has played with chelation—adding protective molecules around the iron—to improve absorption or curb reactivity in tough food matrices. In large-scale use, engineers monitor moisture and oxygen to stop rusting or clumping during storage. Understanding and manipulating these chemical quirks makes or breaks a product’s shelf life and effectiveness.
Shoppers, food formulators, and researchers alike all bump into different names for the same stuff: Iron(II) lactate, E585, and even “lactic acid iron salt.” These terms mostly reflect regulatory conventions around the world. Some regions stick with traditional chemical naming, while food additive codes (like E585) ensure compliance with local food law. As an ingredient in dietary supplements, it may turn up under generic “ferrous salts” or be spelled out by its precise formula. Each synonym sticks to the shared function—bringing easily absorbed iron to the table without overcomplicating the ingredient list or sparking “chemophobia” in label-wary customers.
Iron as a supplement brings real health benefits for folks with anemia or chronic iron loss, but safety thresholds matter just as much. Too much iron, whether from ferrous lactate or any iron salt, can tip over into toxicity—making regulators extra careful about maximum allowed doses in foods. Handling protocols prevent contamination and accidental inhalation during manufacturing, not unlike other fine powders. International guidelines, such as those from the Food Chemicals Codex, keep food and supplement makers in check. Quality assurance teams routinely sample product batches for heavy metals, microbial contamination, and degradation products—so users don't end up swallowing more than they bargained for.
Nutritional fortification takes center stage, where cereals, infant formula, and plant-based beverages rely on ferrous lactate’s solubility and mild taste profile. In pharmaceuticals, it’s a staple for gentle iron replacement therapy—particularly suited to folks who can’t handle gastrointestinal issues from harsher iron forms. Food technologists have also eyed it for its mild oxidation-inhibiting properties, which occasionally hold off unwanted browning in processed fruit products. And while less common, a few niche cosmetic products use it for mild coloring or its skin-conditioning trace minerals. Its importance grows in regions facing widespread iron deficiency, making it a low-cost, effective correction.
Ongoing research circles around bioavailability—scientists want iron that gets absorbed, not just passes through. Clinical studies compare ferrous lactate to ferrous sulfate, iron gluconate, and newer chelates, searching for the gold standard mix of absorption, gentle impact on digestion, and cost. Food scientists study how ferrous lactate behaves in different matrices: Does it affect taste in citrus-based drinks? Does it destabilize colored fruit extracts? Manufacturers explore encapsulation and microgranulation to seal in iron and mask off-notes. Universities and commercial labs measure iron uptake with newer blood markers, zeroing in on which population groups (children, pregnant women, seniors) gain the most from this ingredient.
Concerns always pop up around iron, and ferrous lactate is no exception. Toxicology reports suggest it’s well-tolerated at recommended intake levels, but there’s a razor’s edge between nutritional benefit and overload, especially for young kids who are most at risk during accidental overdoses. Lab animal studies and extensive clinical records suggest its side effect profile lines up with other iron supplements—stomach upset at high doses, nausea, and very high exposures leading to liver stress. The World Health Organization and similar agencies regularly review data to keep guidance in line with the latest science. In practice, labelled warnings and strict dosing protocols anchor prevention.
Looking ahead, interest in ferrous lactate’s role keeps building as both dietary patterns change and iron deficiency stubbornly persists in developed and developing countries. Demand rises in plant-based diets and niche health products, as new research explores ways to improve taste, absorption, and shelf-life. Companies test next-generation forms—microencapsulated, flavor-masked, or partnered with vitamin C and prebiotics. Iron bioavailability continues as a hot research target, and innovating around the delivery matrix or pairing with enhancers promises better results for consumers. The future of food and pharmaceutical science will likely keep ferrous lactate in the spotlight, especially as populations age and dietary fortification remains a frontline health strategy.
Ferrous lactate shows up on the ingredient list of foods like breakfast cereals, canned beans, baby food, and drink powders. It works as a source of iron, which the body needs for healthy blood and proper growth. People often overlook how much iron means for staying energetic and alert throughout the day. Low iron knocks you down—fatigue, paleness, trouble focusing. Looking at the numbers, iron deficiency sits among the top nutritional issues worldwide, especially for women and children. Getting extra iron without changing the taste or look of food helps a lot of people, especially those who can't digest traditional iron supplements.
Working in food labeling and development, I have seen companies struggle with ways to boost nutrition without upsetting customers over color, flavor, or price. Ferrous lactate keeps things simple. Unlike some iron salts, it doesn’t turn foods gray or metallic. That matters for canned vegetables and fruits, where shoppers want vibrant, fresh-looking produce. Packing more iron into a can of beans stops anemia from taking hold, and no one winds up complaining about bitter aftertaste.
Iron supplements fill pharmacy shelves, many of them harsh on the stomach. Patients tell me about nausea or constipation from ferrous sulfate or other common forms. Ferrous lactate works more gently for some. Hospitals treat cases of iron-deficiency anemia with different forms to see what helps people best. Sometimes doctors choose ferrous lactate for kids or elderly patients who don’t handle other iron supplements well.
Scientists produce ferrous lactate by combining lactic acid and iron, often from plant-based or dairy sources. The process supports efforts to use renewable materials and minimize waste. This kind of sourcing means companies offer vegans and vegetarians more options, especially as the demand for plant-based products keeps climbing.
Governments across the world keep a close eye on iron levels in food. The FDA recognizes ferrous lactate as safe for use in foods within established limits. Food safety teams still test for purity and possible contaminants, as with any mineral additive. Too much iron creates risks, especially for people with certain health conditions, so companies follow strict dosing rules.
Many parents don’t realize how hidden iron forms—like ferrous lactate—make nutrition easier for picky eaters. Schools and clinics could do more to educate families on reading nutrition labels, so they don’t fall for empty calories but miss out on essential minerals. Pharmacies and doctors can check whether iron from food or supplements matches each patient’s needs, rather than sticking with a one-size-fits-all approach.
Food science will keep finding new, better ways to sneak nutrition into everyday meals. Ferrous lactate shows how a single ingredient can change lives for those faced with chronic tiredness from iron deficiency. Everyday people, food producers, and medical professionals all shape the story—one package of fortified food at a time.
Ferrous lactate often lands in ingredient lists on breakfast cereals, plant-based meats, or sports drink mixes. It’s a salt made from iron and lactic acid. Companies reach for it because it dissolves easily and boosts the iron content in food. Iron helps the body move oxygen in the blood, and not getting enough can make people feel tired or lightheaded.
Food regulators, including the U.S. Food and Drug Administration and the European Food Safety Authority, have gone over studies on ferrous lactate. They both say it’s safe at the levels found in food. The experts check toxicology data and real-world reports of side effects. Most side effects come from taking too much iron overall, not from ferrous lactate by itself. Sticking to recommended daily iron intake helps people avoid trouble.
Some people worry about chemical names on food packages, especially if they’ve never heard of them. Remember: chemistry doesn’t make something dangerous by default. Table salt has a chemical name (sodium chloride), too. Lactic acid—the other part of ferrous lactate—occurs naturally in many foods, and our muscles produce it during exercise. Both ingredients have a long record of safe use.
Doctors spot iron deficiency pretty often, especially in children and women. Some people, like vegans, athletes, and pregnant women, benefit when foods have added iron. Still, too much iron over time brings its own set of health problems. Hemochromatosis, a rare condition, makes the body store too much iron. For most people, eating foods with added ferrous lactate won’t trigger this. Routine bloodwork helps track iron levels. I worked at a community clinic years ago—people usually got into trouble with iron after taking high-dose supplements, not from their meals.
People sometimes ask if it’s better to pick foods with shorter ingredient lists. Just because a name looks unfamiliar doesn’t mean the ingredient is unsafe. Fact-checking and understanding why something is used helps cut through a lot of confusion. Companies turn to ferrous lactate because it gets iron into foods without turning everything brown or bitter the way some iron salts do. That means more people can get enough iron and enjoy their meals.
My own kids used to hate eating leafy greens or red meat. Fortified bread and breakfast cereal sometimes stood in for spinach or beef at our table, for better or worse. As they got older, we looked for more iron-rich whole foods, but I never worried much about ferrous lactate in their diet. Most nutritionists and doctors I talked with feel the same way.
People who want to avoid unnecessary additives can focus on varied, whole foods. For those who wrestle with iron deficiency, talking with a dietitian or doctor makes sense before starting supplements or making big changes. Reading up on labels, looking past scary-sounding names, and asking questions about nutrition goes a long way. Ferrous lactate, like most food additives approved by health authorities, does its job without causing problems for the vast majority of folks.
Anyone who’s dealt with low iron knows supplements can be a lifesaver. Ferrous lactate lands on pharmacy shelves for a reason: it helps replenish iron in blood, especially for people feeling tired all the time or recovering from blood loss. I've watched family members get their energy back from iron supplements, myself included, after years of fatigue. But adding iron, especially in the form of ferrous lactate, often comes with some baggage.
Most people I talk to complain about their stomach as soon as they start iron pills. Ferrous lactate’s no different. Harvard Health Publishing points out that nausea, stomach cramps, and constipation happen pretty often with iron supplements, ferrous lactate included. Hard stools and abdominal pain show up a lot—some folks practically keep prunes or fiber bars handy because of their iron routine. Sometimes diarrhea rolls in instead, or you wind up with dark-colored stools. Seeing dark stools the first time can feel startling, but it’s a common iron effect and not harmful itself.
Taste can get weird. After taking ferrous lactate, some people start noticing a metallic flavor sticking around in their mouth. It may not sound like such a big deal, but ask anyone who’s dealt with it long-term. Food loses its spark, and even water tastes off. Rarely, canker sores or mouth irritation develop. I’ve seen people quit their supplements early just from these persistent reminders every time they eat or drink.
Every medication, even a mineral, comes with warnings. While not common, allergic reactions do happen. Itching, rash, swelling—these signs show up fast and need urgent care. According to the National Institutes of Health (NIH), kids are especially vulnerable to iron overdose, which can be fatal. Vomiting, rapid heartbeat, or confusion after an accidental double dose needs attention right away. Careful dosing and locked cabinets for supplements keep families safe.
Blood isn’t built to handle endless iron. People with conditions like hemochromatosis absorb too much iron anyway, and more ferrous lactate can do real harm. Symptoms such as joint pain, diabetes, and liver problems may develop from iron overload. Also, iron competes with other nutrients like zinc or calcium, making it harder for the body to absorb what it needs. Medications for thyroid, antibiotics, and antacids also interact with iron absorption.
The simple fixes matter. Taking ferrous lactate with food cuts down on nausea, yet food can block some of the iron your body tries to grab. Splitting the dose or switching to every-other-day dosing can also ease discomfort without losing the benefit, according to recent studies. Drinking plenty of water and increasing dietary fiber softens stools. Honest conversations with your doctor about symptoms or switching iron formulations go a long way—never make supplement changes without them.
Noticing how your body responds helps prevent small side effects from turning into big problems. Tracking symptoms, storing iron safely away from kids, and following prescriptions closely keep risks low. Iron is essential, but taking it isn’t without a tradeoff. Approach ferrous lactate with information, watch for trouble, and lean on healthcare advice for peace of mind.
Plenty of folks trust iron supplements, and ferrous lactate stands out as a source often added to foods. It goes under the radar compared to other iron sources, but what good is a mineral if it changes quality before it reaches your table? Lax handling doesn’t just cost dollars—it can put shelf life, product reputation, and health at risk.
People forget that ferrous lactate brings together iron and lactic acid, both fussy about their surroundings. Iron reacts fast, especially in humid air. Dampness can cause clumping—those familiar rock-hard clods in supplement jars or food premixes. It’s more than a cosmetic problem. Iron reacts with oxygen, forming brownish rust, and the product loses its punch. Add heat to the mix, and shelf life drops even further.
Glass bottles and plastic tubs lined with foil help, but without tight seals or climate control, you’re still running a risk. High humidity doesn’t just sit on the surface. The compound can soak up water like a sponge, and nobody wants a supplement that degrades before its expiration date.
Sunlight—especially UV—breaks down not just vitamins, but iron salts too. Storing ferrous lactate in clear jars on a sunny windowsill speeds up decay. There’s a reason medicine cabinets don’t have skylights. Packing it in amber or opaque containers slows chemical changes, keeping potency longer.
A stuffy warehouse in the summer does no favors for any supplement. High temperature speeds up all kinds of unwanted reactions. Think of it the way you’d treat good olive oil—steady, cool conditions away from appliances and direct sunlight. Below 25°C (77°F) works for almost every shelf-stable supplement, and ferrous lactate fits in that range.
Each batch should list its expiration. Distributors sometimes ignore this, thinking pure chemicals last forever. Regular quality checks make a difference. It’s worth asking suppliers about storage and even testing samples. Over the years, I’ve seen more than one company regret trusting a shipment that spent too long in a shipping container parked outside in the sun.
Home users who keep bottles in a kitchen cupboard can miss this advice, but a drawer away from the stove and out of the light keeps things simple. For factories, a good rule is to dedicate storage areas with humidity controls, even simple silica gel packs or basic air conditioning, and keep containers tightly sealed at all times.
Some of the best results I’ve seen come from storage audits: just walking through the warehouse every few weeks, feeling containers, checking the air, and tossing anything stored poorly. This hands-on approach might not create headlines, but it saves hassle and protects everyone who counts on safe, reliable supplements.
Poor storage isn’t just about waste or lost money; it means less reliable nutrition, which matters most for those who depend on iron-fortified foods. By keeping things dry, cool, and out of direct light, both manufacturers and home users can trust that ferrous lactate stays safe and effective from factory to fork.
Ferrous lactate shows up in ingredient lists for foods such as canned beans, breakfast cereals, and nutritional supplements. It gives products a boost of iron and keeps food looking fresh with its color-fixing powers. Some folks avoid animal products for ethical or environmental reasons and want to know if this ingredient fits with their choices. The answer depends on where ferrous lactate comes from, how it’s made, and what brands tell their customers.
Lactic acid forms the backbone of ferrous lactate. It’s a common misconception that lactic acid always comes from animals because of the name. Most of the time, food and supplement companies create lactic acid through fermentation. They use carbohydrates from beet sugar, corn, or other plant sources. Fermentation relies on bacteria or fungi. There’s no meat or dairy needed here; even vegan supplement companies use plant sources. Still, some lactic acid could link back to milk sugar (lactose), especially if a company cuts corners or uses old-school methods.
Ferrous iron comes from mineral sources. The iron itself doesn’t tie directly to animal products, so it slides easily into vegetarian and vegan regimens—assuming the processing doesn’t involve animal-based agents.
Labels rarely give the whole backstory. Food companies only have to list ferrous lactate, not where they got their lactic acid or iron. Some bigger brands answer emails about this or explain sourcing on their websites. Vegan certification and vegetarian labels give peace of mind, but some companies add those only when customers make a fuss. I have called up supplement makers myself for this reason. Once or twice, a customer service rep still had to “check with the lab.” This shows that clarity doesn’t always come easily, and it pays to check with the producer, even if it means digging through websites or picking up the phone.
A person who eats vegetarian or vegan for personal or ethical reasons might worry about even trace animal products sneaking into food. Iron supplements get a lot of play in vegan diets since plant-based foods don’t always offer enough. Poor iron intake can leave people tired and anemic. If someone picks up a supplement or cereal to fill the gap, those small details in sourcing become pretty important. For me, getting straight answers from brands is just as much about respecting animal welfare as it is about my own health. These questions about ferrous lactate turn into bigger ones about transparency and ingredient traceability in the world of packaged foods.
Full disclosure helps everybody. Certifying bodies like the Vegan Society or Vegetarian Society set the bar high, and their stamps stand out for consumers. They push companies to back up marketing with solid evidence. Clearer ingredient sourcing on packaging—something beyond vague catchphrases—would go a long way. Also, public-facing documents like certificates of analysis, supply chain audits, or FAQ sections could clear up confusion without long email chains. Customers could then choose food and supplements with confidence, knowing that both health and values align.
| Names | |
| Preferred IUPAC name | iron(II) 2-hydroxypropanoate |
| Other names |
Iron(II) lactate Ferrous 2-hydroxypropanoate Iron lactate Lactic acid iron(II) salt |
| Pronunciation | /ˈfer.əs ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 5905-52-2 |
| Beilstein Reference | 87892 |
| ChEBI | CHEBI:75829 |
| ChEMBL | CHEMBL1201532 |
| ChemSpider | 12211 |
| DrugBank | DB13872 |
| ECHA InfoCard | 03db979d-cb6b-4c3a-a652-681bb012a84b |
| EC Number | 299-128-4 |
| Gmelin Reference | 82686 |
| KEGG | C01797 |
| MeSH | D003670 |
| PubChem CID | 2723636 |
| RTECS number | OG8225000 |
| UNII | 90E5XF03E0 |
| UN number | UN9206 |
| CompTox Dashboard (EPA) | DTXSID5020669 |
| Properties | |
| Chemical formula | C6H10FeO6 |
| Molar mass | 289.96 g/mol |
| Appearance | Light greenish-white crystalline powder |
| Odor | Odorless |
| Density | Density: 0.8 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.5 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.6 |
| Basicity (pKb) | 3.7 |
| Magnetic susceptibility (χ) | −49.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.435 |
| Dipole moment | 2.69 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 208.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1617.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1439 kJ/mol |
| Pharmacology | |
| ATC code | B03AA08 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302: Harmful if swallowed. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. Do not ingest. |
| NFPA 704 (fire diamond) | 1-0-0-W |
| Autoignition temperature | > 400 °C |
| Lethal dose or concentration | LD50 (oral, rat): 3,250 mg/kg |
| LD50 (median dose) | LD50 (median dose): 3,250 mg/kg (rat, oral) |
| NIOSH | WN3675000 |
| PEL (Permissible) | 0.2% |
| REL (Recommended) | 10-20 mg/kg |
| IDLH (Immediate danger) | IDLH not established |
| Related compounds | |
| Related compounds |
Ferrous fumarate Ferrous gluconate Ferrous sulfate Iron(III) lactate |