People have experimented with mineral fortification for over a century, seeking not only better nutrition but also safer food processing methods. Iron lactate entered the scene as a direct answer to iron deficiency in both food fortification and pharmaceutical applications. Its roots reach back to discoveries in biochemistry connecting iron’s role in hemoglobin with poor public health. Scientists saw that adding bioavailable iron to staple foods could combat anemia. Early methods for producing iron lactate tapped into basic organic acid reactions, combining lactic acid with iron salts. Over decades, production scaled from chemical curiosities to reliable, standardized supplies. Research into its absorption and interaction with other food components defined its use in bakery, dairy, and drink production across Europe, Asia, and North America.
Iron lactate usually takes the form of a light yellow or pale green, almost odorless powder, known for dissolving in water with mild effervescence. It serves as an iron supplement, color retention agent, and pH stabilizer, especially in foods targeting iron-deficient populations. Manufacturers market it under names like Ferrous Lactate, Iron(II) lactate, and E585, used across food, supplements, and even brewing for color protection. The product's appeal often ties to its relatively high biocompatibility and mild taste, making it a candidate for direct addition without overwhelming flavor or odor, which can plague other iron salts.
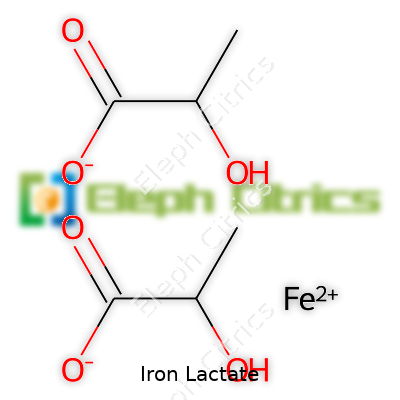
Pure iron lactate registers a molecular weight around 233.96 g/mol (dihydrate), with a chemical formula of C6H10FeO6·2H2O. It will not stand up to strong light or prolonged exposure to air, where color can shift due to oxidation. The powder easily absorbs moisture. Its solubility in cold water simplifies mixing into beverages and dough, bypassing gritty sediment or unpleasant texture. The pH of a 1% water solution lands between 3.5 and 4.5. Unlike ferrous sulfate, its taste is more subtle and less metallic, allowing wider applications in food, especially where flavor sensitivity matters.
Technical data sheets for iron lactate spell out purity requirements, iron content (usually 18%-24% metallic Fe), lead and arsenic limits, and trace element thresholds. Food-grade products must meet specifications issued by authorities like the FCC, USP, or EU food additive regulations. Batch certificates often confirm microbial safety and absence of harmful solvent residues. Ingredient panels identify it as ferrous lactate or E585, especially in Europe. In supplements, legal frameworks dictate strict phrasing concerning source, yield, and daily value contribution. Labels advise against dosing for infants except on medical advice, tying into regulatory warnings for vulnerable consumers.
Industrial production of iron lactate starts with lactic acid fermentation, yielding lactic acid at high purity. Technicians introduce ferrous carbonate, ferrous sulfate, or other iron(II) salts to the solution, maintaining mixing under inert gas to protect these iron ions from air oxidation. The resulting liquid quickly forms iron lactate, which they then evaporate, crystallize, and dry—often under vacuum. Steps to prevent ferric iron (Fe3+) conversion matter because higher oxidation states degrade bioavailability and darken color. Each plant tweaks process parameters for yield, particle size, and purity, optimizing for customer demand.
Iron lactate owes its success in part to the chemical stability of the iron(II) ion when paired with lactate. Exposure to oxygen gradually triggers oxidation to ferric iron, producing off-colors and possible loss of nutritional benefit. Blenders sometimes include ascorbic acid (vitamin C) in finished foods or supplements to stabilize the iron in its +2 state. In some products, iron lactate serves not only as a supplement but also as a gentle reducing agent, helping set color in cured meats or fried snacks. Researchers monitor side reactions closely, particularly in fortified dairy, where ongoing Maillard reactions (browning due to protein-sugar interaction) can influence appearance and taste profiles.
The chemical goes by several designations, including Ferrous Lactate, Iron(II) lactate, Lactic Acid, Iron(2+) Salt, and internationally as E585. Pharmacopeia and food codex entries list it under its IUPAC name, but supplement labels usually prefer everyday terms like “iron (from iron lactate).” Some older manuals and trade catalogs might still call it Iron L(+) Lactate, reflecting the lactic acid stereochemistry used from fermentation.
Handling protocols for iron lactate look similar to those for other powdered minerals. Facilities rely on dust control, food-safe gloves, and robust cleaning regimens to prevent cross-contamination. Material Safety Data Sheets (MSDS) highlight risk from inhalation dust, especially in bulk transfer or mixing. Chronic exposure can irritate the respiratory tract, though acute toxicity is rare at occupational levels. GMP (Good Manufacturing Practice) requirements dictate meticulous lot tracking and allergen management. Food and supplement facilities test incoming raw material for heavy metals, pathogens, and strictly monitor batch blending to ensure accurate dosing, since iron can build up to toxic levels with careless overuse.
Food manufacturers use iron lactate for more than just nutrient boosts. Bakery mixes, breakfast cereals, and fruit drinks often owe their mineral claims to this compound. Ready-to-eat meats showcase its effect on cooked color and shelf stability. In plant-based beverages, iron lactate helps shore up nutritional value without changing taste or masking natural flavors. Brewers lean on its mild color-protecting traits for stouts and wheat beers. Pet food companies rely on it as a digestible iron source with lower risk of metallic “off” notes. Nutraceutical and pharmaceutical firms formulate tablets, soft gels, and multivitamin syrups targeting populations from pregnant women to seniors with higher iron needs.
Research on iron lactate follows two main threads: bioavailability in fortified foods and reactivity under storage or heat stress. Nutritionists have published dozens of studies comparing its absorption rates against other iron compounds, showing slightly lower but steadier intake versus ferrous sulfate in some models. In reformulated foods, food scientists test it for impact on texture, flavor, and antioxidant preservation. Pilot-scale bakeries and beverage makers often run head-to-head trials with iron gluconate or chelates like NaFeEDTA, weighing cost-benefit tradeoffs. Chemists try to improve iron stability using microencapsulation and pH buffers, searching for ways to lock in beneficial properties without tipping into unwanted browning or iron-catalyzed spoilage.
Over the decades, scientists have run toxicity trials in rodents, in vitro gut cell cultures, and controlled clinical settings. Most findings agree: at recommended consumption levels, iron lactate produces no mutagenic or carcinogenic effects. Acute overdose, as with any iron compound, causes gastric distress and—at high doses—potentially life-threatening hemochromatosis. Young children face the highest accidental overdose risk because iron tablets can resemble candy. Regulatory reviews by the World Health Organization (WHO), US FDA, and EFSA consistently rank iron lactate as a safe “generally recognized as safe” (GRAS) ingredient for intended use levels, provided strict quality control and consumer guidance.
Looking ahead, iron lactate faces mounting demand as global health efforts ramp up iron fortification in low- and middle-income countries, where deficiencies remain stubborn. New applications begin surfacing in dairy alternatives, immune-support beverages, and clinical nutrition products tailored for malnutrition. With plant-based diets on the rise, formulators search for iron sources that don’t bring metallic taste or cause silt in drinks—and iron lactate stands out. Investments in microencapsulation, flavor masking, and next-generation iron “salt” hybrids aim to plug remaining absorption gaps and slow shelf-life degradation. As synthetic biology advances, cleaner production routes with waste minimization and lower carbon footprints get closer, promising a molecule with both legacy and untapped unrealized value for food, pharma, and nutrition science.
Iron keeps us going. For people like me, who eat a lot of grain-based foods and less meat, getting enough iron is a daily concern. Iron lactate steps in as a reliable way to increase iron levels without a strong metallic taste or gritty texture. This iron compound shows up quietly in cereal, baby food, and flour. It absorbs well in the gut, which means kids and adults get more out of every bite. Public health programs in several countries recommend iron fortification to avoid anemia. I've seen how tiredness fades and focus returns for people who get the iron they need. The World Health Organization recognizes the huge impact of iron additives like iron lactate in reducing global iron deficiency.
I love a good jar of olives. Their crisp color doesn’t happen by accident. Food producers use iron lactate in specialty foods—like green olives—to set and stabilize color. This process means the olives look brighter and fresher on the shelf. The chemical reacts with natural pigments, helping maintain that honest green hue people expect. Without this step, olives can turn an unappetizing brown, and honestly, who wants that on a dinner table?
Doctors frequently recommend iron for patients with low red blood cell counts. Iron lactate can be found in some multivitamin tablets and liquid formulas. Unlike ferrous sulfate, iron lactate is easier on the stomach. I've met folks who'd stopped taking iron completely due to side effects—switching to gentler forms of iron reopened the door to better health for them. Maltodextrin and iron lactate combined create supplements that dissolve quickly and work for people who can't handle other iron salts. Pregnant women and folks with kidney conditions may benefit from these blends.
Not just humans face iron issues—farmers look for ways to give livestock the best start. Iron lactate shows up in piglet and poultry feed, supporting growth and vitality. Young animals raised on big farms often lack outdoor access, so they miss out on nutrients naturally present in soil. Iron-fortified feed means healthier animals and better yields. Healthier livestock lead to fewer veterinary costs and better food products down the chain.
Iron lactate doesn't change the way bread rises or cakes look. Bakers add it directly to dough, and the iron disperses evenly, meaning a family pulling bread from the oven gets nutrition baked into their daily staple. Unlike harsher iron salts, it keeps bread soft and leaves no aftertaste. At home, I’ve seen more and more baking mixes with added iron—especially in products aimed at children or people with restricted diets.
Food safety bodies in the United States and Europe keep a close eye on iron lactate use. Food scientists have studied it for years, confirming that ordinary intake levels pose no risk for healthy people. For those with hemochromatosis—where the body loads up on too much iron—careful labeling helps prevent unintentional misuse.
Iron lactate isn’t flashy, but its role in keeping communities thriving is easy to miss. People feel energized, children grow, and food looks and tastes better—all thanks to this overlooked ingredient. By supporting its careful use and raising awareness through public health outreach or clearer food labels, we can help more people get the nutrition their bodies rely on.
Iron lactate goes into everything from breakfast cereals to plant-based foods. This compound helps people get more iron, especially those who don't eat red meat or can’t absorb iron from other sources. Doctors talk to patients about iron often, and picking the right supplement sometimes gets confusing. Some options bother the stomach or cause constipation. Iron lactate usually does not. Its mild flavor and good absorption make it popular with both food producers and supplement makers.
Research has looked at how the human body processes iron lactate. Both the FDA in the United States and the European Food Safety Authority reviewed iron lactate and found no sign of harm at usual intake levels. A standard adult rarely gets anywhere near the suggested upper tolerable intake from diet and supplements combined. Typical side effects — if someone takes much more than needed — may include upset stomach, nausea, or darker stools. Those effects show up with almost any form of added iron.
As long as people use iron lactate inside the recommended range, no evidence links it to chronic conditions or long-term health problems. One important thing: those with hemochromatosis, a condition that makes the body hold onto too much iron, should talk with a medical expert before taking any iron source. For the vast majority, iron lactate does its job and leaves the system like it should.
Not every product on the shelf meets the same standard. Some cheaper supplements from unreliable companies could be contaminated with heavy metals or carry less iron than the label shows. To trust what goes into your body, look for brands that earn third-party certifications like NSF or USP. These groups test for contaminants and confirm label accuracy. Reputable food manufacturers follow strict rules, and iron lactate from these sources lines up with what food safety agencies consider safe.
Over the years, my time with patients and research teams showed me that the source of your supplement matters as much as the ingredient itself. Patients who chose supplements based on price alone sometimes ended up with products that didn't improve their blood values or made them feel worse. Once they switched to known brands with quality tests, their experience changed for the better.
Too little iron causes fatigue; too much can cause damage. Most adults, especially women of childbearing age, need a steady supply. Iron lactate gives the body what it needs in a form absorbed without as many side effects. Expecting mothers or those with specific health issues should check with a doctor before adding any supplement, including this one. Personalized advice prevents both iron overload and deficiency.
Iron lactate works best when combined with a meal that includes vitamin C. This simple pairing, like drinking orange juice or adding bell peppers to your plate, can make a difference in how well iron absorbs. Too many people still deal with fatigue and low energy caused by iron deficiency, which the World Health Organization calls one of the most common nutrition issues worldwide. More attention to iron, paired with safe forms like iron lactate, would cut these numbers.
Safe use of iron lactate starts with knowing your needs, picking trusted products, and talking to a health care provider before making changes. The science supports its use as a safe and effective way to meet iron needs for most people.
Tiredness creeps in, you feel like you never catch up on energy, and lightheaded spells become a regular thing. These are common signs when iron runs low, something more people experience than they might think. Doctors spot this problem most often with blood tests, and treating it quickly makes all the difference. The food industry, nutritionists, and parents face the challenge head-on every day. Getting enough iron isn’t always easy, especially for kids, teenagers, and women of childbearing age. Many aren’t keen on the chalky taste or stomach troubles that come with typical iron supplements, and this is where iron lactate stands apart.
Iron lactate brings an answer rooted in both science and daily life. You’ll find it in cereals, flour, drinks, and other fortified foods. My own family used to struggle with the taste of iron syrups prescribed for anemia—no one looked forward to that morning spoonful. Iron lactate sidesteps most flavor issues, working seamlessly in juices, dairy, sports bars, or even bread. Research backs this up. A study in the journal Food Chemistry highlights iron lactate’s mild taste and solid solubility, making it more practical for food fortification than swinging for the usual ferrous sulfate, which often leaves a harsh, metallic bite.
Absorbing iron well often means fewer side effects. Many iron pills can cause stomach cramps or constipation, stopping folks from finishing their course. Iron lactate tends to land softer in the stomach, offering a better option for people who give up on harsher supplements. I’ve seen teens stick to courses that use iron lactate much more consistently, and their blood counts reflect that reliability.
People need real solutions that fit their routines. Fortifying everyday foods with iron lactate gives a quiet boost without extra effort or unpleasant tastes. Backpack snacks, school lunch biscuits, and simple breakfast cereals can play a stronger role in keeping iron stores up, especially when families already face busy schedules. It’s about lowering barriers so more people get the nutrients they need without having to overhaul their diet.
This flexibility supports broader public health goals too. Health agencies point to iron deficiency as the world’s most common nutritional disorder. Studies show that areas with food fortification programs using gentle, well-absorbed forms like iron lactate see stronger results in lowering rates of anemia. Companies respond to the call for better fortification tools. Their products reach farther when they use ingredients that blend into foods and drinks kids and adults already love.
Iron lactate’s safety gets support from food authorities like the European Food Safety Authority and the FDA, who have evaluated it based on evidence. Researchers measure how much of the mineral the body takes in, how it interacts with other nutrients, and its long-term safety. These checks keep consumers in the loop and make the ingredient a trusted tool for dietitians and food makers.
Progress shouldn’t just mean fancier packaging or more marketing buzz. It comes from listening to people, testing what works, and showing results that last beyond the first trial. Iron lactate represents that spirit—a solution shaped by research, daily experience, and the ongoing need for better, more accessible nutrition. For families, food producers, and caregivers, it’s a practical tool that answers real needs.
Iron lactate never grabbed headlines like iron pills or fortified breakfast cereals, yet it keeps showing up in ingredient lists on everything from sports drinks to kids’ vitamins. I remember trying it myself after a doctor caught my sluggish iron levels during a yearly checkup. The pitch sounded straightforward — a gentler, more palatable iron source than the gritty tablets that had upset my stomach before. But just like any supplement or food additive, iron lactate asks for a closer look, especially with folks seeking it for regular use.
Iron keeps your blood moving, your mind sharp, and your body from feeling run down. Iron lactate delivers this mineral in a form that’s supposed to sidestep the common problems that chase people away from iron pills. More than one nutritionist told me the lactate salt offers better absorption than basic ferrous sulfate and triggers fewer complaints about constipation.
Yet, my experience lined up with what medical research says: even “gentle” iron supplements can cause trouble. Gas, stomach pain, and nausea popped up at times, especially if I took iron lactate without food. Friends have reported that some brands taste metallic or leave them queasy if they’re on an empty stomach. Scientists point to the same list — the National Institutes of Health says pretty much every iron supplement creates some form of digestive upset in certain people, whether the label reads “easy on your gut” or not.
Most people bounce back from mild side effects without lasting harm, but some risks can’t be ignored. Iron toxicity isn’t new, and it doesn’t play favorites with the compound you use. Too much iron — through supplements, especially — can damage organs and even threaten life, especially in kids who swallow extra tablets by accident. According to Poison Control, iron is a leading cause of poisoning deaths in children under six.
Allergic reactions appear less often, yet hives or trouble breathing after taking iron lactate call for immediate medical help. Folks with conditions like hemochromatosis (iron overload) risk severe harm from even small doses, so no new iron supplement belongs on their shelf at all.
Living with low iron can drag you down, but chasing a quick fix without real knowledge rarely pans out. Bloodwork should shape any decision about iron, not a guess or something overheard at the gym. Doctors recommend routine checks if you’re using iron lactate long-term. They point out that food-based iron — beans, lean meats, spinach — doesn’t bring these same risks if you get enough through diet.
Whenever someone wants a supplement, I suggest watching out for any new stomach changes and tracking them. Taking iron lactate with meals made a difference for me, dampening the gut churn. I also learned to start with the smallest effective dose and skip vitamin C megadoses unless my doctor actually says I’m missing it.
Iron lactate fills a gap for people who can’t meet iron needs with food alone but brings its own baggage, from mild digestive problems to rare, serious outcomes. Trust in bloodwork, honest conversations with professionals, and a habit of listening to your own body. These steps keep the benefits of iron supplementation real and the risks under control.
Iron acts as a critical building block for our bodies. It gives us stamina, supports brain power, and keeps our immune systems sharp. Iron deficiency hits harder than most think, draining energy and focus, even affecting the way kids learn. A shortage of iron slips quietly into life, often showing up as total exhaustion or the feeling that climbing stairs is tougher than it should be. Doctors pay attention to iron levels, especially in kids, women of childbearing age, and vegetarians, since these groups tend to run low more frequently.
Iron lactate steps up as a solid choice for people aiming to restore depleted iron. You can find it in supplements and certain fortified foods. Most folks assume more iron means better results, but the body doesn’t work that way. Excess iron stores itself in organs, raising the risk for liver issues and other health problems. You want to get it right—enough to replenish, but not so much it creates trouble.
The typical adult dose for supplemental iron falls between 50-100 mg of elemental iron daily. Iron lactate provides about 19% elemental iron by weight. That translates to somewhere around 300 mg of iron lactate to get 57 mg elemental iron. Kids need less—children in the age group of 4 to 8 years usually require 10 mg of elemental iron daily, and teens or adolescent girls may need up to 15 mg due to growth and menstruation. For them, measuring the right iron lactate amount matters even more, and it’s better to go with a healthcare professional’s recommendation rather than guess.
There’s never a good reason to start guessing when it comes to iron. Iron overload can slip up on you, especially for people with hereditary hemochromatosis or chronic health conditions. Doctors check ferritin and hemoglobin with blood tests before recommending supplements. Even if fatigue, pale skin, or restless legs scream “iron deficiency,” self-treating without lab results sometimes makes matters worse. Also, some common pills carry other minerals that block or help iron absorption. Vitamin C, for example, actually boosts iron’s impact in the body.
Iron supplements sometimes lead to stomach upset or constipation. Drinking plenty of water, taking iron with meals, or using slow-release tablets cuts down on most problems. Coffee, tea, and calcium drop absorption, so keep them two hours apart from doses. Pregnant women especially need precise amounts; too little hurts the baby, too much leads to side effects. Medical guidance gets even more important in these cases.
Folks living with chronic illnesses or following restricted diets (like vegans) need to double-check dosing with a clinic or nutritionist. If supplements look necessary, pick products from respected brands, since purity and content matter a lot. Symptoms that don’t clear up after a few weeks of taking iron supplements should prompt another visit to the doctor. Sometimes, the body absorbs some forms of iron better than others.
Raising public awareness about how to spot and treat iron deficiency is a job for healthcare workers, schools, and even food makers. Simple solutions like using iron-fortified foods, cooking in cast iron, or pairing dietary iron with vitamin C-rich fruits prevent deeper problems. Worry less about blanket solutions and more about tuning care to each person’s needs, history, and unique body chemistry. Lab work plus conversations with medical professionals always guide the way.
| Names | |
| Preferred IUPAC name | Iron(II) 2-hydroxypropanoate |
| Other names |
Ferrous lactate Iron(II) lactate Lactic acid, iron(2+) salt E585 |
| Pronunciation | /ˈaɪərn ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 585-23-9 |
| Beilstein Reference | 1132657 |
| ChEBI | CHEBI:61375 |
| ChEMBL | CHEMBL1201532 |
| ChemSpider | 22995 |
| DrugBank | DB11345 |
| ECHA InfoCard | ECHA InfoCard: 03-2119474445-39-0000 |
| EC Number | 299-128-4 |
| Gmelin Reference | 7875 |
| KEGG | C01708 |
| MeSH | D019364 |
| PubChem CID | 53477782 |
| RTECS number | OI3530000 |
| UNII | 51YO4D7I36 |
| UN number | UN3260 |
| CompTox Dashboard (EPA) | DTXSID0026933 |
| Properties | |
| Chemical formula | C6H10FeO6 |
| Molar mass | 289.96 g/mol |
| Appearance | Light yellow or yellow-green crystalline powder. |
| Odor | Odorless |
| Density | 1.5 g/cm³ |
| Solubility in water | Soluble |
| log P | -3.55 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.5 |
| Basicity (pKb) | 8.0 |
| Magnetic susceptibility (χ) | Paramagnetic |
| Refractive index (nD) | 1.58 |
| Dipole moment | 2.3 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 159.0 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1567.3 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1364.7 kJ/mol |
| Pharmacology | |
| ATC code | B03AB05 |
| Hazards | |
| Main hazards | Not hazardous according to GHS classification. |
| GHS labelling | GHS07, Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0-NFPA |
| Lethal dose or concentration | LD50 (oral, rat): 819 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 3,260 mg/kg |
| NIOSH | WT2932500 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 10 mg (as iron)/day |
| IDLH (Immediate danger) | No IDLH established. |
| Related compounds | |
| Related compounds |
Calcium lactate Magnesium lactate Manganese(II) lactate Zinc lactate |