The rise of isopropyl lactate in chemical and industrial circles comes as no accident. This ester traces its lineage back to fermentation discoveries in the early chemical era, powered by the attempts to convert waste agricultural byproducts into building blocks for solvents and lubricants. Decades ago, chemists turned their sights to lactic acid esters as a way to bridge green chemistry with industrial performance. With lactic acid first isolated from sour milk in the 18th century, the modern chemical synthesis advanced dramatically in the mid-20th century, with a strong push for bio-based solvents as petroleum prices and environmental pressure mounted. As the laboratory ledgers shifted, isopropyl lactate found a niche—less volatility than ethyl acetate, better solvency than methyl lactate, fewer regulatory headaches than chlorinated alternatives, dawning as a preferred choice for both formulators focused on worker safety and manufacturers staring down stricter emissions laws.
Isopropyl lactate arrives today as a clear, nearly odorless liquid, offering a smart alternative to more hazardous solvents. Labs often value it for its gentle evaporation and the fact that it sidesteps that sharp solvent tang. Those working on skin-contact formulas or electronics appreciate that it keeps volatility and irritation low. The main places you see it—cleaners, personal care, resins—show the versatility people always look for in solvents. The demand for this ester comes not from some passing fad, but a real-world need for smart, safe replacements in increasingly regulated environments.
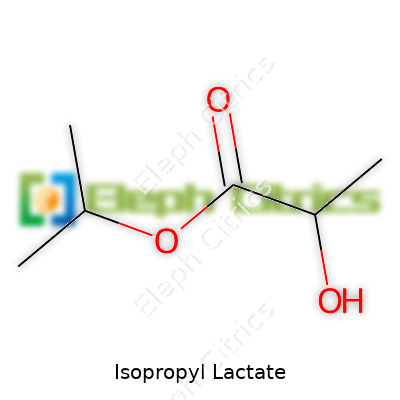
Sitting in the bottle, isopropyl lactate hardly calls attention to itself: colorless, neutral smell, decent water solubility. Its molecular formula, C6H12O3, and a molecular weight of 132.16 g/mol, keep things straightforward. Boiling takes place around 180°C so it resists running off during mild heating. Viscosity sits in a manageable range, which means it moves well through pipes and mixing lines, neither watery nor sticky. The flash point sits in safer territory, above what most standard room temperatures threaten, keeping handling routines simpler. Due to the alcohol and ester groups, chemists can further tweak its properties, making it a good template for modifications.
Product purity often matters more in daily practice than it seems. For isopropyl lactate, reputable producers keep purity above 98%. Water content and acid number, though they might bore on a spec sheet, matter hugely—water above 0.2% raises questions about shelf life and downstream reactivity. Containers range from one-liter lab bottles to bulk drums, always marked with batch numbers, hazard pictograms, and details for emergency handling. The proper hazard labels come from the Globally Harmonized System, and anyone working with this ester takes seriously the need for clean labeling, proper PPE, and well-ventilated storage.
Fixing lactic acid to isopropanol under acid catalysis sits at the core of manufacturing isopropyl lactate. Acidic conditions push the reaction forward, usually with dehydration to pull water formed during esterification and get good yields. The type of catalyst—be it sulfuric acid or a more selective resin—affects impurity levels, separation work, and even environmental scorecards. Many commercial facilities recover and recycle unreacted alcohol to boost efficiency, aligning with today’s push for green chemistry. The leftover water finds use as a cleaning agent or gets treated before disposal, closing the loop and limiting waste generation.
Isopropyl lactate reacts predictably for those who spend their days in synth labs. Its primary reactivity centers around the ester linkage, sensitive to both strong acids and bases. Hydrolysis breaks it down—back to lactic acid and isopropanol—making it a good candidate for formulations needing eventual biodegradation. Chemists build on this backbone, creating derivatives with tailored solubility, evaporation, or even antimicrobial properties. The secondary alcohol backbone stands as a handle for transformations, offering a platform for attached functional groups or further esterification.
Looking through product catalogues feels like walking through a thesaurus—synonyms abound. Commercial orders list isopropyl 2-hydroxypropanoate, isopropyl α-hydroxypropionate, or sometimes just 2-hydroxypropanoic acid isopropyl ester. Cosmetic ingredient labels swap in INCI names, while chemical distributors may add trade names or proprietary blends. Mismatches in naming often frustrate those hunting supply chain alternatives, but ultimately the industry leans on these synonyms to cut through confusion and communicate clearly across borders and regulatory frameworks.
From a safety perspective, isopropyl lactate fits neatly into well-understood solvent categories. Handling advice goes beyond paperwork—users pay attention to skin and eye contact due to potential mild irritation. Proper gloves and goggles stand between comfortable use and unnecessary accidents. Given its moderate flash point, fire risk remains manageable, but vapor buildup in enclosed spaces makes a solid ventilation plan critical. Spill cleanup tends to be straightforward, usually relying on absorbent materials, with disposal consistent with local environmental standards. Workers who know their solvents respect safety datasheets, not out of fear, but from the years of experience that say a few minutes reading today prevents lost time injuries tomorrow.
Mainstream applications for isopropyl lactate grow from the practical demands of both industries and households. In personal care, this ester works as an emollient and solvent in creams due to gentle touch and easy spread. Pharmaceutical manufacturers use it as a vehicle for drug delivery, where quick skin absorption without harshness matters. Electronic cleaning sees it as an alternative to acetone—getting delicate parts spotless without corroding or leaving residue. The printing and coatings sector likes it for faster drying inks, and plastics manufacturers value its compatibility in biodegradable blends. Now, food processors rarely turn to it due to regulatory caution, but safety data keeps that possibility open for tomorrow’s regulation shifts.
Researchers keep pushing the boundaries on what esters like isopropyl lactate can do. Recent efforts focus on renewable sourcing, using lactic acid generated from plant-based feedstocks and promoting closed-loop reuse of solvents during synthesis. Scientists also explore blending isopropyl lactate into antimicrobial coatings and medical adhesives, pulling from the compound’s low toxicity and ready hydrolysis. Work in green chemistry journals points to more energy-efficient production, recyclable catalysts, and even enzymatic synthesis routes, which cut waste by skipping harsh reagents. As industries look toward safer and more sustainable practices, R&D teams target isopropyl lactate for broader applications in specialty chemicals, greener cleaning products, and healthcare devices.
Every chemical on the bench gets scrutinized for possible harm. Toxicologists working on isopropyl lactate cite low oral and dermal toxicity in standard models; skin irritation remains mild and typically reversible. Inhalation exposure rarely causes problems, and the breakdown products—lactic acid and isopropanol—rank as familiar, manageable substances in the body. That said, flooding cell cultures or fish tanks with a solvent never makes sense, so wastewater treatment works to break down any traces before releasing effluent. Chronic toxicity studies stay ongoing, mostly to reassure regulators and the public about large-scale use. In my experience, the vigilance of safety teams and their commitment to personal training make workplace overexposure unlikely, but any new application triggers a re-examination of long-term health data.
The next decade looks promising for isopropyl lactate, driven by the push for cleaner, safer, and greener solvents. As regulations get stricter, especially in Europe and North America, more users look to esters that meet both industrial performance and environmental goals. Markets for biodegradable plastics, safe cosmetics, and precision cleaning open new doors, while consumer awareness fuels demand for labels that spell out every ingredient in plain language. Green manufacturing methods, renewable sourcing, and transparent supply chains increasingly shape purchasing decisions. Growth will come from companies ready to invest in new production technologies, close the loop on their waste, and back up safety claims with real data—not just promises. For those of us invested in better chemistry, isopropyl lactate stands as both a benchmark of progress and a call to keep pushing for safer, cleaner alternatives.
Isopropyl lactate doesn’t get much attention outside of chemical circles or product manufacturing, but its fingerprints show up everywhere: lotions, shampoos, household cleaners, even some medicines. This simple compound, a mix of lactic acid and isopropyl alcohol, works behind the scenes to improve how products feel and function. In my own routine, I’ve run into it plenty—especially while testing new skin creams or comparing deodorants on drugstore shelves. Its effectiveness always popped out to me from the way a lotion would actually penetrate, not just sit on my skin.
Manufacturers consider isopropyl lactate a gem because it tackles multiple challenges at once. On one hand, it acts as a solvent, breaking down other ingredients so they blend nicely into a silky, balanced formula. Personal care products—moisturizers, sunscreens, antiperspirants—gain from how it helps actives dissolve and spread. Greasy or sticky residue often disappears with isopropyl lactate in the recipe, which makes a difference for anyone who dislikes the heavy feeling of some creams. Over the years, I’ve noticed more brands call out “fast absorbing” or “non-greasy,” and this compound often plays a part.
In cosmetics and skincare, absorption matters. Dermatologists point to better skin health when moisturizers actually penetrate. Scientific reviews back it up: isopropyl lactate delivers emollients where skin cells need them, instead of leaving a film on the surface. I’ve talked with formulators who lean on it to reduce the drag of thick creams, making products pleasant to use and less likely to clog pores.
Outside of beauty, isopropyl lactate steps up as a cleaner and carrier in household products. It softens fanatical residues left by makeup or sticky labels. Hospital staff and pharmacists know it as an ingredient in topical medicines that require quick skin absorption. When you rub on an anesthetic gel that vanishes without a trace, this compound made it possible. Years ago, while speaking with a rehab nurse, I learned about the challenges of formulating pain relief creams for elderly patients—getting medication into tired joints hinges on the right carrier, not just active drugs.
Safety always lands on top of the list. Review panels and regulatory agencies see isopropyl lactate as safe in standard concentrations. Overuse may upset sensitive skin, like many solvents, but responsible brands market patch tests or allergy warnings. Environmental impact raises questions about biodegradability and runoff. Most data reports it breaks down fairly quickly, posing less risk than many harsher synthetics. Companies gravitate toward these greener profiles as shoppers get savvier about what lands in drains and landfills.
As ingredient lists grow longer and labels pile up new claims, consumers chase products that do better for skin and the planet. To move forward, more transparency about ingredient sources would help. Pressure from watchful shoppers drives manufacturers to publish not just what goes in a product, but why it’s there and how it leaves the environment. Honest testing, ongoing safety research, and easy-to-read packaging steer the market. My own experience picking up bottles and reading tiny print taught me the value of clear ingredient information.
In the end, isopropyl lactate works quietly, improving daily products in practical ways. Thoughtful sourcing, honest communication, and ongoing safety checks will keep it a helpful tool in the kit—not just for chemists, but for anyone reaching for something that works and feels right.
It’s found in a variety of cosmetic products—creams, lotions, sunscreens, aftershaves, and even some antiperspirants. Isopropyl lactate’s ability to soften skin leaves many asking whether it’s safe for daily use. Skincare manufacturers look for ingredients that glide onto the skin and absorb quickly without clogging pores, and isopropyl lactate ticks those boxes. Yet real safety isn’t just about a product feeling nice. It’s about how it interacts with real people, with real skin.
Dermatologists and toxicologists have spent years keeping an eye on cosmetic ingredients. Panels like the Cosmetic Ingredient Review (CIR) have actually looked at isopropyl lactate’s track record. After sifting through both laboratory tests and user experiences, the experts didn’t find evidence that the ingredient causes major side effects in most people using it as intended. According to published research, the molecule breaks down on the skin into isopropyl alcohol and lactic acid, both common in beauty products.
Lactic acid gets plenty of use in skincare for its ability to exfoliate, and isopropyl alcohol appears in lots of surface-cleaning products. Most healthy adults won’t see irritation from isopropyl lactate in concentrations found in cosmetics. In fact, reported problems tend to involve overuse or exposure to broken skin. The ingredient doesn't build up in the body or linger on the skin for long. For folks with strong allergies, the story can be different. Some people react to alcohols or esters, and no skincare product is a guaranteed match for everyone.
I have sensitive skin myself and tend to read ingredient lists with a critical eye. From my experience, products containing isopropyl lactate never set off the kind of redness or burning that sometimes comes with harsh solvents. Many friends working in cosmetics and healthcare use products containing it without worry. For most, it just glides on and soaks in, leaving no residue. It’s even considered non-comedogenic, so it won’t join the ranks of common pore-blockers.
Skin safety depends on more than just one ingredient. Product formulas matter. Clean manufacturing processes and storage play a big role too. The best manufacturers put ingredients like isopropyl lactate through stability and allergy checks before reaching the shelves. Product labels should highlight concentrations and any respected safety ratings. Smart shoppers don’t rely on marketing claims alone—they take a minute to research and choose brands committed to both safety and honesty. Patch testing on a small area always makes sense if you’re new to a product.
Some worried voices in the skincare community call for more studies, especially for people with eczema, psoriasis, or other chronic skin issues. Doctors often remind their patients that moderation is crucial, not just for actives like isopropyl lactate, but for any unfamiliar skincare ingredient. If a product stings, causes swelling, or brings out redness, stopping use straight away and seeing a dermatologist makes all the difference. Healthy skin routines grow from knowledge and paying attention to your body’s own signals.
You might spot isopropyl lactate on ingredient labels for lotions, creams, and even some personal care sprays. Companies like it because it helps creams feel smoother and less greasy. In my experience working in a pharmacy, people rarely asked questions about it, probably because the name sounds tame compared to big words like “hydroquinone.” Still, just because something is common in products doesn’t mean we should ignore its downsides.
Most stories of trouble with isopropyl lactate start with skin reactions. People with sensitive skin often notice redness, irritation, or a feeling of burning. I’ve talked with regular lotion users who gave up on certain creams because of rashes that wouldn’t quit. Scientists supporting these stories point to cases where contact dermatitis shows up after using products with isopropyl lactate.
People with eczema or allergies might need to keep an eye out, since broken skin soaks up chemicals much more easily. A study from the International Journal of Toxicology listed skin sensitization among the most common issues. These symptoms show up more often in people who already have trouble with harsh soaps or fragrances.
Using a product made with too much isopropyl lactate, or putting it on damaged skin, can let it get deep enough to cause real trouble—kind of like how an open cut stings from just a little dab of rubbing alcohol. Some manufacturers even warn against using it on wounds or large patches of skin for this reason. There’s not a pile of reports about deeper absorption leading to systemic problems, but it remains a possibility, especially with kids or people with very thin skin.
Nobody wants breathing trouble from a beauty routine. Inhaling the vapors from products containing isopropyl lactate can bother the lungs, especially if used in a small, closed room, or by someone with asthma. Even some experienced hair stylists have talked about nose and throat irritation after working with sprays that include this chemical.
Isopropyl lactate breaks down into isopropyl alcohol and lactic acid. Both of those can make their way into the water stream if washed down the drain in large amounts. Water safety research hasn’t pinned this as a crisis, but every extra chemical dumped into the environment adds up. People might shrug this off, but over time, wastewater treatment plants see more challenges from chemical runoff, especially as our medicine cabinets get more crowded.
Some simple habits go a long way. Patch testing—a tiny dab on a small patch of skin first—helps people spot irritation before it spreads. Reading ingredient lists becomes even more important for folks with allergies or sensitive skin. If a rash pops up, switching to fragrance-free and additive-free skincare makes a difference. Dermatologists often suggest avoiding any new cosmetic product that lists unfamiliar chemicals if you’ve had allergic problems before.
It also helps if authorities require clearer ingredient labeling. More transparency keeps people from walking blind into reactions they could have dodged. Sharing the facts helps everybody make safer choices for themselves—and keeps everyone, pharmacists included, having more pleasant conversations about skincare.
Moisturizers, creams, body lotions—these products rely on ingredients that don’t just sit on the skin’s surface. Isopropyl lactate shows up in cosmetic formulas for a good reason. It’s an ester that comes from lactic acid and isopropyl alcohol, making it a useful solvent and emollient. The idea here: draw out smoothness, help the skin feel less greasy, and deliver a lightweight texture that many folks prefer.
Plenty of cosmetic chemists use isopropyl lactate to create that soft, spreadable finish in skin-care formulations. It helps thin thick lotions and keeps creams from feeling heavy. My experience with dry skin led me to lotions packed with heavy butters—great for deep winter, terrible for daily use under a shirt. Something lighter works better, and isopropyl lactate often joins the formula to provide that.
People worry about what goes on their skin, and I get that—skin absorbs more than we realize. Cosmetic safety panels like the Cosmetic Ingredient Review (CIR) and the European Commission’s Scientific Committee on Consumer Safety (SCCS) keep tabs on these additives. Their findings on isopropyl lactate show it doesn’t irritate most skin types in normal cosmetic amounts. It’s not classified as a carcinogen or mutagen. I’ve checked regulatory lists, and isopropyl lactate has global approval for use in rinse-off and leave-on products, with typical concentrations ranging between 0.1% and 3%.
That said, the skin does its job as a barrier, so the risk of significant systemic exposure seems low. Allergic reactions can happen, as with anything, but reports stay low and isolated. For anyone with sensitivities, patch testing always seems smart, especially when trying out a new active ingredient.
These days, a growing group of shoppers scans ingredient lists for anything unfamiliar or chemical-sounding. I’ve chatted with people who won’t buy a product if it lists anything they can’t spell. Some believe synthetic ingredients always spell trouble, but the story isn’t so black and white. Isopropyl lactate doesn’t appear anywhere near the top of “red flag” lists from safety watchdogs. Brands using it should keep communication open, explain the sourcing, and highlight the safety evidence.
Clean beauty pushed the industry to re-examine old formulas. Companies echo the demand for transparency now, and that’s good for everyone. Anyone involved in formulating—or just buying skincare—benefits from knowing what ingredients do and how they stack up in terms of safety. I like to see brands post easy summaries on their websites; it gives buyers the power to make their own calls.
Cosmetics can work well with a blend of natural and synthetic ingredients. Isopropyl lactate adds something valuable to a formulation, especially for people seeking lightweight options or a silky skin feel without heavy oils. The science so far offers reassurance on its safety record, and regulatory bodies haven’t flagged it for risk when used as intended.
For brands, updating product labeling, encouraging consumer feedback, and supporting independent safety reviews help make sure product choices remain safe and trusted. Anyone worried about reactions should test a small patch of skin before jumping to full use. As always, keeping up with ongoing research and consumer outreach keeps the cosmetic world moving in a safer, more transparent direction.
You see these names pop up on product labels all the time. Isopropyl lactate and isopropyl alcohol sound pretty similar, and that gets a lot of folks wondering if they’re really just the same thing. I’ve heard this question from college friends trying to ace their chemistry midterms, and from parents puzzled over skin-care ingredients. Yet they serve completely different purposes, especially once you look beyond their chemical roots.
Isopropyl alcohol calls to mind the familiar smell of rubbing alcohol, something you reach for to clean out a scrape or wipe down a sticky countertop. It’s a small molecule, technically a simple alcohol, and it evaporates fast—one of the reasons it feels so cool on your skin. Hospitals and home first-aid kits rely on it because it kills germs, evaporates clean, and works in cleaning solutions. You find it in everything from disinfectant wipes to household glass cleaners.
Isopropyl lactate doesn’t get as much attention in drugstore aisles, but it crops up plenty in cosmetics and lotions. Chemically, it’s an ester: it comes from combining lactic acid with isopropyl alcohol. This change matters. Instead of smelling sharp and evaporating in seconds, isopropyl lactate acts more like an oily liquid. It softens the skin, helps blend other ingredients, and gives that silky feeling you notice in moisturizers and aftershave.
Mixing up these two chemicals could bring up all sorts of issues. If someone poured isopropyl lactate into a wound to clean it, the results would be disappointing and potentially cause irritation. It doesn’t disinfect like isopropyl alcohol. Flip things around—swapping isopropyl alcohol for isopropyl lactate in a moisturizer—and skin might end up dried out, even raw. These two ingredients bring completely opposite effects despite their similar names.
I remember as a teen believing that anything with that harsh “alcohol” smell could be used the same way, even though my hands stung for ages after using rubbing alcohol for everything from bug bites to acne. It took reading ingredient lists and talking to pharmacists to learn where that line gets drawn—and that rubbing alcohol is not a skincare miracle worker.
Isopropyl alcohol is widely used as a disinfectant, and the Centers for Disease Control and Prevention recommends solutions of 70% for general disinfection purposes. Experts recommend keeping products that contain isopropyl alcohol away from broken or sensitive skin unless a healthcare provider has said otherwise. Isopropyl lactate, on the other hand, shows up in ingredient lists for moisturizing creams and makeup removers for its emollient and solvent qualities, according to safety data published by the U.S. Food and Drug Administration. Both substances are considered safe when used in proper amounts for their intended purposes, but they must not be confused.
Part of the problem comes down to labeling and ingredient education. Ingredient names in personal care and home care products can lead to a lot of head-scratching if skin safety or disinfection are at stake. Packaging designed with clear, transparent ingredient lists—and even brief explanations—would help. Pharmacists, doctors, and science educators can bridge these knowledge gaps for families juggling dozens of chemical names.
Health-conscious shoppers and parents want to keep their families safe. Taking time to learn which ingredients match which routines should be part of building that safety net. Skipping the guesswork by checking reputable sources and talking to professionals can spare a lot of frustration and keep skin and health protected.
| Names | |
| Preferred IUPAC name | Propan-2-yl 2-hydroxypropanoate |
| Other names |
Isopropyl 2-hydroxypropanoate Propane-2-yl 2-hydroxypropanoate Isopropyl alpha-hydroxypropionate |
| Pronunciation | /ˌaɪ.səˈproʊ.pɪl ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | [630-17-1] |
| Beilstein Reference | 1821404 |
| ChEBI | CHEBI:31215 |
| ChEMBL | CHEMBL416162 |
| ChemSpider | 12143 |
| DrugBank | DB11124 |
| ECHA InfoCard | 03b88d1e-b98c-4912-9e1c-c8d7fc8d2e7e |
| EC Number | 211-902-1 |
| Gmelin Reference | 120865 |
| KEGG | C18599 |
| MeSH | D016680 |
| PubChem CID | 7332 |
| RTECS number | OJ1750000 |
| UNII | RG7D1AP8PR |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C6H12O3 |
| Molar mass | 162.19 g/mol |
| Appearance | Colorless liquid |
| Odor | pleasant, fruity |
| Density | 0.95 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | 0.74 |
| Vapor pressure | 0.08 mmHg (25°C) |
| Acidity (pKa) | 15.3 |
| Basicity (pKb) | 15.30 |
| Magnetic susceptibility (χ) | -8.41×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.426–1.430 |
| Viscosity | 12 cP (25°C) |
| Dipole moment | 2.62 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 348.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -566.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2325.7 kJ/mol |
| Pharmacology | |
| ATC code | D02AX01 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02, GHS07 |
| Signal word | Warning |
| Hazard statements | H317, H319 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P312, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 88 °C |
| Autoignition temperature | 399 °C |
| Explosive limits | Explosive limits: 1.2–8% |
| Lethal dose or concentration | LD50 (oral, rat): 2900 mg/kg |
| LD50 (median dose) | 5 g/kg (rat, oral) |
| NIOSH | WJ3325000 |
| PEL (Permissible) | 50 ppm |
| REL (Recommended) | 1.02 mg/kg bw |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Methyl lactate Ethyl lactate Butyl lactate Isopropyl alcohol Lactic acid |