Lauryl lactate did not spring out of nowhere. Chemists and formulators searched for ingredients that marry mildness with functionality, tracing the origin of lauryl lactate to the broader story of lactic acid esters. Early days focused on extracting lactic acid from sour milk, which dates back centuries thanks to fermentation. Much later, when the 20th-century industrial push demanded milder surfactants and specialty emulsifiers, manufacturers began to esterify lactic acid with fatty alcohols like lauryl alcohol. This blend of natural origin and tailored chemistry gave the world a new tool for personal care and industrial applications. A background in cosmetic science helps place lauryl lactate as part of a move toward greener, safer, and skin-friendlier formulas—an effort kicked into higher gear with regulatory and consumer awareness taking off after 1970.
Lauryl lactate, also known as dodecyl lactate, delivers more than just cleansing or softening power. The molecule, formed by the reaction between lauryl alcohol and lactic acid, fits right into personal care routines, food production, and even plastics processing. Whether looking for a skin-conditioning agent, a mild emulsifier, or something that brings slip to a lotion, this ester finds a place. Its blend of natural lactic acid and a 12-carbon fatty chain results in an ingredient used in shampoos, creams, detergents, and sometimes as a specialty solvent or plasticizer for PLA (polylactic acid) production. Folks in the lab appreciate that it plays well with common cosmetics ingredients and doesn’t introduce major formulation headaches.
Lauryl lactate appears as a colorless to pale yellow oily liquid at room temperature, thanks to the moderate chain length of its fatty alcohol component. The viscosity feels smoother than water, providing that “glide” in personal care products. Its molecular weight sits around 258 g/mol, and it carries a melting point slightly below room temperature, making it pourable and easy to blend. In terms of solubility, don’t expect it to mix straight into water, but it disperses well in alcohols, oils, and other esters. A slightly sweet, fatty scent comes natural, without overwhelming the senses. Compared to shorter-chain lactic esters, its longer hydrophobic tail makes it less volatile and easier to handle in bulk.
Manufacturers supply lauryl lactate at high purity—typically over 98%. Modern labeling requirements mean you will see the INCI (International Nomenclature of Cosmetic Ingredients) name “Lauryl Lactate” on personal care products, or an E number (E482b) when permitted in food. The CAS number reads 6283-92-7. Physical specs remain tightly controlled: low acid value, minimal color (often tested with the Gardner scale), and low odor are industry musts. Many suppliers also track moisture content, ensuring shelf stability. Labs run checks using gas chromatography, confirming identity and absence of major impurities, as regulatory oversight extends into both cosmetics and foodstuffs.
Production often relies on a direct esterification route. Lactic acid and lauryl alcohol combine in a reactor vessel with a mild acid catalyst, often under reduced pressure to pull off the water formed during the reaction. Temperatures climb to 120–150°C, pushing the equilibrium in the right direction. The resulting mixture gets neutralized, washed to remove any unreacted acids, and then distilled to isolate pure lauryl lactate. Scale ranges from laboratory synthesis for R&D to multi-tonne batches in chemical plants. Some manufacturers use enzymatic esterification under milder conditions, tapping into the growing demand for bio-based, sustainable ingredients. Workers with plant experience know how tightly operators monitor pressure and temperature throughout, with distillation columns designed to handle the volatility and recovery of unreacted starting materials.
Besides straightforward esterification, lauryl lactate is involved in transesterification reactions, letting chemists swap its lauryl group for other alcohols to create designer esters. Under alkaline conditions, the molecule undergoes saponification, reverting to lauryl alcohol and lactic acid. This comes in handy for certain controlled-release or biodegradable plastics. Chemical treatment also allows for functionalization, such as sulfation, opening up routes to surfactants with unique foaming or mildness properties. From a researcher’s view, lauryl lactate can serve as a feedstock for synthesizing derivatives used in specialty cleaning, advanced cosmetics, or even bio-lubricants. These modifications drive innovation across sectors looking for safer, greener solvents and plasticizers.
Lauryl lactate goes by several names: dodecyl lactate, lactic acid dodecyl ester, or, less commonly, lactic acid, dodecyl ester. Marketing and technical datasheets sometimes label it with trade names. Whether browsing a bulk chemical catalogue or an ingredient list on a bottle of hair conditioner, the name usually reflects its structure, giving consumers and formulators confidence in what they are getting. International trade aligns around clear CAS numbers and INCI naming, keeping regulatory reporting straightforward.
Regulators take a keen interest in the safe handling of lauryl lactate, especially in products used on skin and in foods. Toxicology studies report low acute toxicity, but formulators must avoid excessive use in leave-on skin products to minimize sensitization risk. Technical data sheets highlight the need for gloves and goggles in bulk handling, as with most concentrated esters, and recommend good ventilation to reduce vapor exposure—even if its odor profile doesn’t seem harsh. For finished products, patch testing helps confirm safety for sensitive users. Cosmetic ingredient review panels in the US (CIR) and Europe (SCCS) have combed through the data, typically approving lauryl lactate for rinse-off and leave-on applications, so long as purity and maximum use levels are respected.
In cosmetics and personal care, lauryl lactate works as an emollient, mild cleanser, and lubricant. Anyone who has mixed up a batch of lotion or shampoo will appreciate how it creates a smooth, spreadable texture and a comfortable after-feel. This ester enhances solubilization of fragrances and other oil-soluble actives. Beyond beauty products, food processors sometimes harness its emulsifying and surface-active features, though use remains limited by regulatory frameworks. Plastics manufacturers incorporate lauryl lactate as a plasticizer or processing aid in polylactic acid (PLA) and other biodegradable polymers. Its biodegradability, drawn from the lactic acid backbone, attracts attention from sustainability advocates aiming to curb persistent plastic waste. Lubricant formulators exploit its fatty chain for better slip agents, appealing to industries trying to phase out conventional petroleum-based additives.
R&D in lauryl lactate continues to focus on greener production methods, such as bio-catalysis and enzymatic synthesis, which slash energy inputs and reduce unwanted byproducts. Chemists work on tuning the physical and sensory properties of personal care formulas, blending lauryl lactate with other esters to hit specific viscosity or absorption targets. Environmental testing pushes its limits in degradability and ecotoxicity, with data often showing favorable breakdown in soil and water. Some researchers explore lauryl lactate-based systems as vehicles for targeted delivery of skincare actives, thanks to its affinity for skin lipids. Others, in the food space, look for new regulatory approvals and expanded uses as part of preservative blends. Advanced plasticizers built on lauryl lactate structure capture the attention of scientists designing next-generation compostable packaging.
Studies indicate that lauryl lactate has a relatively low level of acute and chronic toxicity. Laboratory assays track minimal oral and dermal toxicity at concentrations used in cosmetics and food. Long-term studies, both in vivo and in vitro, rarely flag skin irritation or sensitization, especially compared to harsher esters or surfactants. Still, some users may show mild irritation with repeated or high-dose exposure, leading to safety thresholds in formulation. Regulatory reviews by agencies such as the FDA in the United States or EFSA in Europe underscore the need for high purity and careful control of reaction byproducts—no one wants residual lauric acid or unreacted alcohol. Environmental fate research also suggests lauryl lactate degrades well, with low bioaccumulation risk.
Lauryl lactate stands poised to grow its footprint as consumer preferences veer toward natural-sourced, gentle, and effective ingredients across skincare, food, and plastics. Plant-based feedstocks promise to anchor its sustainability story, particularly as biorefineries mature. Technical advances in enzymatic synthesis aim to cut production costs and carbon emissions, unlocking new supply chains. As more countries tighten plastic waste regulations, biodegradable polymers will need effective, green plasticizers like lauryl lactate to perform. R&D teams will continue exploring tailored derivatives for use in pharmaceuticals, agriculture, and specialty coatings. The shift toward simple, transparent ingredient labeling favors the inclusion of straightforward molecules like lauryl lactate. As regulatory frameworks catch up with innovation, lauryl lactate looks set to remain a versatile building block in the bio-based economy.
Chemicals with complicated names show up in everyday products, and Lauryl Lactate happens to be one of them. For anyone who checks product labels in drugstores or supermarkets, this ingredient stands out in lotions, creams, and even hair conditioners. It’s not one of those substances that only scientists talk about; people have interactions with it almost daily without realizing, so it makes sense to learn what it’s doing in these products.
Most people want skin to feel smooth, clean, and hydrated. Lauryl Lactate comes into play here. It helps condition the skin, often making lotions and creams glide on more easily and absorb better. Dermatologists talk about how it improves a product’s feel and consistency. This hands-on change in texture matters because nobody likes sticky, greasy, or rough skin after using a moisturizer.
I remember using some heavy-duty hand lotions in the winter, and all too often, hands would feel slick long after application. Then I tried a lotion that blended in quickly and left zero residue. Checking the label, Lauryl Lactate was in there. This kind of personal experience is echoed by many, and research backs it up—Lauryl Lactate reduces heaviness in creams and can help products spread better over the skin.
Lauryl Lactate serves as more than a skin conditioner. Picture a product loaded with ingredients that do great things for the skin—vitamins, hydrators, barrier protectors. These ingredients need a good base to reach where they’re supposed to work. Lauryl Lactate acts almost like a traffic director, helping the other ingredients move and distribute through the skin’s upper layer. In a technical sense, it has mild penetration-enhancing action. So, those antioxidants and moisturizers actually do more when paired with Lauryl Lactate.
Cosmetic scientists, like those at the Personal Care Products Council, have found Lauryl Lactate can also gently remove old skin cells. Nobody wants harsh scrubs every day, so products with this ingredient can give a subtle, regular exfoliating effect, which over a few weeks helps create a fresher skin surface.
Shampoos and conditioners also make use of Lauryl Lactate. People try new haircare products hoping for that smoother, more manageable finish right after the shower. This ingredient helps detangle, soften, and tame frizz. No fancy jargon needed—the hair feels better, and hairdressers notice the difference.
Safety always comes up with ingredients nobody can pronounce at first glance. Regulatory agencies in the US, Europe, and Asia have all given Lauryl Lactate the green light at current usage levels. At home, parents want peace of mind over what they put on their kids' skin, so this assurance carries weight.
Lauryl Lactate is partly plant-based, made from lauryl alcohol (coconut or palm sources) and lactic acid (a natural fermentation product). Scientists are still debating the best ways to make these processes more sustainable. The rise of ‘clean beauty’ means companies keep a closer eye on ingredient sourcing and manufacturing impact.
Lauryl Lactate’s role is crystal clear: it helps skin and hair feel better by improving product texture and performance. Smart shopping means checking labels, knowing what works for your own needs, and choosing brands that value clear communication about their ingredients and environmental impact. The next time a label lists Lauryl Lactate, there’s a story behind it—one that brings a better sensory experience and supports the goal of effective, comfortable care.
Walk into any pharmacy and you’ll see moisturizers, cleansers, and hair conditioners lining the shelves. Flip a few bottles around—Lauryl Lactate shows up all over the place. This ingredient acts as a gentle exfoliant and helps skin feel smoother. In my own bathroom cabinet, I’ve noticed it in at least two cleansers and one body lotion. If your skin ever felt a little softer after using one of these products, odds are this ester had a hand in it.
Lauryl Lactate comes from lauryl alcohol and lactic acid, two compounds commonly found in personal care products. Lactic acid itself has a strong track record as an alpha hydroxy acid (AHA), a family of mild exfoliants. According to studies reported by the Cosmetic Ingredient Review Expert Panel, Lauryl Lactate does not show signs of significant skin irritation at concentrations used in skincare products. After digging into published reports, I saw that most cases of redness or mild stinging linked to Lauryl Lactate involved people already sensitive to AHAs.
My dermatologist once explained that while lactic acid on its own can sting or strip moisture when used in high doses, Lauryl Lactate is less aggressive. Scientific reviews back this up: in trials with healthy adults, Lauryl Lactate used at concentrations up to 10% in leave-on creams rarely triggered irritation. In rinse-off formulations, it poses even less risk since exposure time drops.
Sensitive skin types may still react to even mild acids, including derivatives like Lauryl Lactate. People with rosacea, eczema, or recent sunburn probably want to avoid products loaded with exfoliants. In my own experience with eczema as a teen, even gentle ingredients could sometimes surprise me with redness. While Lauryl Lactate is mild for most, it’s not a guarantee for everyone. Patch testing a new product before slathering it on makes a difference—after all, everyone’s skin responds a little differently.
Regulators keep a close eye on ingredient safety. The U.S. Food and Drug Administration (FDA) lists lactic acid and its derivatives, including Lauryl Lactate, as ingredients considered safe for use in cosmetics. The European Commission's Scientific Committee on Consumer Safety echoes this, placing Lauryl Lactate in the low-risk category when it comes to potential health effects. No major bans or restrictions exist in regions like the United States, Canada, or the European Union.
A quick scan of major skin care brands shows that Lauryl Lactate sits near the middle or end of the ingredient list—usually a hint that concentrations stay low. People with healthy, balanced skin rarely face trouble using it daily, especially when they follow up with moisturizer and SPF. Washing off any new product after a short test run on your inner wrist or behind your ear avoids surprises.
Concerned about over-exfoliation? Combining Lauryl Lactate lotions with other strong AHAs or retinoids can sometimes tip the balance, leaving skin prone to flaking or irritation. Spacing out exfoliants and adding calming ingredients—ceramides, glycerin, panthenol—can help. Dermatologists often say a simple approach beats complicated routines stuffed with actives.
At the end of a long day, feeling confident in your skincare routine counts for a lot. If Lauryl Lactate keeps your skin clear and comfortable, there's little reason to worry. Still, listening to your skin—just like reading nutrition labels—builds the best habits for the long run.
Lauryl lactate pops up on ingredient lists of skincare, shampoos, and even some food-grade lubricants. Those keeping an eye out for “natural” labels have wondered about its origins. Lauryl lactate comes from lauryl alcohol and lactic acid. Lauryl alcohol often gets sourced from coconut or palm oil, while lactic acid forms during the fermentation of sugars – usually from corn or beet sugar.
Standing in the personal care aisle, I always check what goes on my skin. If it says “plant-derived,” I feel a bit better. Lauryl lactate checks that box for most suppliers. From my research, large chemical manufacturers take lauryl alcohol, strip it from natural oils, and combine it with lactic acid. The lactic acid side often comes straight out of a fermentation process – think of what happens when making yogurt, except done on a much bigger scale.
That’s not to say every lauryl lactate on the market comes from sustainably-grown coconuts or organic tapioca syrup. Sometimes manufacturers turn to synthetic sources if using natural ones gets too expensive. Not every bottle of lotion using lauryl lactate can claim a totally plant-based backstory without proper verification.
Product origin matters to shoppers with allergies, dietary restrictions, and those conscious of sustainability. My cousin, who avoids animal products and tries to limit her environmental impact, picks up products only when she’s sure the ingredients follow those same values. She once wrote to a brand to check if their lauryl lactate came from palm oil, a crop linked to deforestation. With more consumers asking, companies now publish sourcing details or certifications like RSPO (Roundtable on Sustainable Palm Oil) and Non-GMO Project.
There’s a broader debate about “natural” versus “synthetic.” Some argue synthetic ingredients mean fewer pests, lower crop use, and steadier supply. On the other hand, genuine plant-based sourcing appeals to consumers looking for fewer processing steps and a closer tie to nature. Both approaches have value, but transparency clears up confusion.
Spotting lauryl lactate on packaging doesn’t tell the whole story. Labels, especially in the U.S., rarely list whether the source comes from renewable crops or fossil fuels. If you want a natural origin specifically, only trust brands willing to share supply chain details. Publicly available third-party certifications help too. The Environmental Working Group, for instance, rates ingredients for safety and origin, although thoroughness varies by ingredient.
I once called a cosmetics company to ask about their lauryl lactate, and after a few emails, their chemist confirmed it had coconut oil roots, not petroleum. It only took an afternoon, but it made all the difference for my peace of mind. Shoppers shouldn’t have to go on a fact-finding mission every time they buy something. Companies would do better to make these details easy to find.
A growing chunk of consumers want ingredients from known, clean sources. Brands gain loyalty by providing clear traces from farm to product. Industry groups could help by pushing for stricter labeling on derivatives like lauryl lactate. Retailers, too, can make it easier to shop with values by demanding better info from suppliers. In the world of personal care and food, what goes unnoticed today can become tomorrow’s big selling point. Ingredient origin matters – not just for science, but for trust.
Lauryl lactate shows up in a lot of personal care products. You find it on the ingredient list for lotions, shampoos, and skincare creams. It works as an emollient and a skin conditioning agent. It comes from lauryl alcohol and lactic acid, both of which are found in nature. Since it helps skin feel smooth and doesn't leave much residue, formulators like using it across a range of items.
No matter how gentle an ingredient seems, someone can react badly to it. Lauryl lactate isn’t high on the list for allergic outbreaks, but reports still surface in dermatology literature. The main issue? Lauryl lactate acts as an ester, and the body may recognize it as a foreign molecule. For some people, that can mean trouble in the form of redness, itching, or hives.
For those with sensitive skin or a history of eczema, the risk increases. Studies from the American Contact Dermatitis Society mention ester-based emollients as rare but possible triggers. I’ve heard patients in dermatology clinics talk about breakouts or stinging after using new moisturizers or shampoos; sometimes, after patch testing, lauryl lactate turns out to be the cause.
The fact remains: anything can be an allergen to someone. Lauryl lactate is no exception. Its use in “hypoallergenic” products may sound reassuring, but anyone who’s struggled to find skin comfort knows that labels don’t guarantee a reaction-free experience.
People living with allergies or skin sensitivities need confidence about what they put on their skin. Ingredients like lauryl lactate get used in products meant for babies and adults alike. If an ingredient can throw the skin out of balance, even at rare rates, people deserve clear information about those risks.
According to the FDA and European Medicines Agency, reports of lauryl lactate allergies remain low. Still, as more people begin using complex formulations or applying multiple products at once, the risk of developing sensitivities seems to be rising. Even low-risk ingredients cause real problems for real people.
Dermatologists stress the importance of patch testing new products. It doesn’t take long—dab a small amount behind the ear or inside the elbow, then watch for a reaction over a couple of days. That approach costs little and prevents uncomfortable flare-ups for many people, myself included.
Manufacturers should provide full ingredient transparency. Instead of featuring catch-all claims like “gentle” or “dermatologist tested,” listing out every component helps consumers make real decisions. Some companies offer sample packs or travel sizes. That helps cut the risk of investing in a product only to have to toss it after a single use.
Doctors and pharmacists provide guidance for anyone concerned about allergic reactions. If a rash or burning pops up after using something new, unused product samples come in handy for patch testing and pinpointing the culprit.
Plenty of people handle lauryl lactate without issue. A small percentage react, though, and for that group, even rare reactions feel huge. Everyone deserves respect for what their body tells them. Listening to those signals, sharing reliable ingredient data, and learning from real-world stories protects everyone who wants healthy skin.
Standing in the drugstore aisle, most people never give much thought to the ingredients listed on the back of a lotion bottle. For those of us with sensitive skin, the details matter. Lauryl lactate pops up in many personal care items, showing up in cleansers, creams, and even baby products. But slick marketing doesn’t answer the real question—does it sit well with sensitive complexions?
Lauryl lactate acts as an emollient. That means it helps soften and smooth dry patches. Many manufacturers like it for its gentle exfoliating punch—since it breaks down in the skin, lactic acid is released and helps loosen old, rough surface cells. On paper, it sounds perfect for delicate skin that flakes or feels raw.
That’s not the whole story, though. Some skin types react badly to even mild acids. In a way, it depends on concentration and what else a product contains. I learned this the hard way after trying a “mild” moisturizer filled with lauryl lactate. My face stung red for days. Only after talking with my dermatologist did I realize that not every gentle ingredient works for everyone.
According to the Cosmetic Ingredient Review, lauryl lactate generally counts as safe for the skin at typical concentrations seen in over-the-counter products. It’s even cleared for use in products meant for babies. Lactic acid—its breakdown result—exists naturally in our bodies and forms part of the skin’s natural moisturizing factor, which helps keep everything balanced.
Dermatologists often say that lauryl lactate tends to cause fewer problems than harsh surfactants or strong alpha hydroxy acids. Still, on ultra-sensitive skin, even the mildest acid will sometimes trigger a rash, redness, or increased dryness. Condition, not just the ingredient, can flip the switch from safe to irritating.
People living with eczema, rosacea, or frequent redness know one wrong move in their skin routine spells trouble. I’ve seen plenty of forums where users praise lauryl lactate for gentle exfoliation that doesn’t make skin peel or burn. Others, though, share cautionary tales: redness, itching, breakouts. No single answer exists for everyone.
Patch testing before adding anything new helps avoid disaster. A tiny dab behind the ear or under the jaw tells the truth quicker than any influencer review. My takeaway after a few bad reactions—dermatologists really mean it when they say to test first.
If you’re shopping for products with lauryl lactate and worry about your skin, pick brands that list full ingredient breakdowns and skip strong fragrances or added alcohols. Fragrance free usually means fewer chances for irritation. Better yet, ask for product samples and try out patch tests at home.
Consulting a dermatologist before a big switch goes a long way. These professionals read ingredient labels like chefs read recipes and can steer you clear of ingredients that might look fine but work poorly for your skin. Sensitive skin is unpredictable. Experience—your own and others’—tends to teach better than any label ever could.
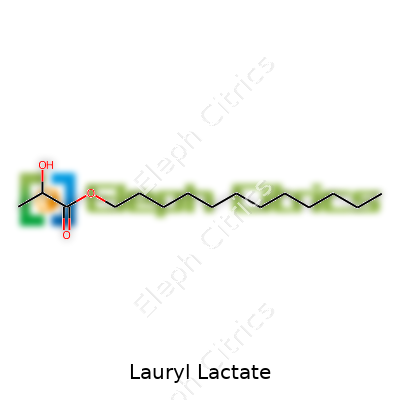
| Names | |
| Preferred IUPAC name | dodecyl 2-hydroxypropanoate |
| Other names |
Lauric acid lactic acid ester Dodecyl lactate Lauryl 2-hydroxypropanoate |
| Pronunciation | /ˈlɔːr.ɪl ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 18682-55-6 |
| 3D model (JSmol) | `/www.rcsb.org/3d-view/5ZUX?representation=ballAndStick&as=JSmol` |
| Beilstein Reference | 1207212 |
| ChEBI | CHEBI:39299 |
| ChEMBL | CHEMBL143709 |
| ChemSpider | 94530 |
| DrugBank | DB11362 |
| ECHA InfoCard | 03daa25f-eeb1-4142-b5ba-6c15edc2f397 |
| EC Number | 211-435-0 |
| Gmelin Reference | 8935 |
| KEGG | C14319 |
| MeSH | D019228 |
| PubChem CID | 87755 |
| RTECS number | OJ8750000 |
| UNII | 92T5T60S2B |
| UN number | UN number: "UN3272 |
| Properties | |
| Chemical formula | C15H30O3 |
| Molar mass | 258.384 g/mol |
| Appearance | Clear colourless liquid |
| Odor | Faint, pleasant |
| Density | 0.95 g/cm3 |
| Solubility in water | Insoluble |
| log P | 2.92 |
| Vapor pressure | <0.1 mm Hg (20°C) |
| Acidity (pKa) | pKa ≈ 3.86 |
| Basicity (pKb) | 12.48 |
| Refractive index (nD) | 1.436 |
| Viscosity | 75 mPa·s |
| Dipole moment | 2.64 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 421.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -756.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -7892.6 kJ/mol |
| Pharmacology | |
| ATC code | D11AX20 |
| Hazards | |
| Main hazards | Causes serious eye irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | Wash thoroughly after handling. Wear protective gloves/eye protection/face protection. IF ON SKIN: Wash with plenty of water. If skin irritation occurs: Get medical advice/attention. Take off contaminated clothing and wash it before reuse. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | > 112 °C |
| Autoignition temperature | 355°C |
| Lethal dose or concentration | LD50 (oral, rat): > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): 2800 mg/kg (rat, oral) |
| NIOSH | GV5950000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 8.5 |
| Related compounds | |
| Related compounds |
Lauric acid Lactic acid Sodium lauryl sulfate Ethyl lactate Stearyl lactate |