Lithium lactate stepped out of the shadows around the late nineteenth century, catching the attention of early chemists keen on understanding metal-organic salts. It followed on the heels of discoveries about lithium carbonate’s effects, which landed in medical textbooks due to its role in mood disorder treatment. Over decades, the curiosity about how lithium bonds with organic acids like lactic acid grew, and researchers focused on what unfolds when such compounds enter biological or industrial processes. Laboratories tinkered with lithium ions and lactic acid’s properties, asking what happens in the body and in manufacturing, which laid the foundation for modern uses across multiple sectors.
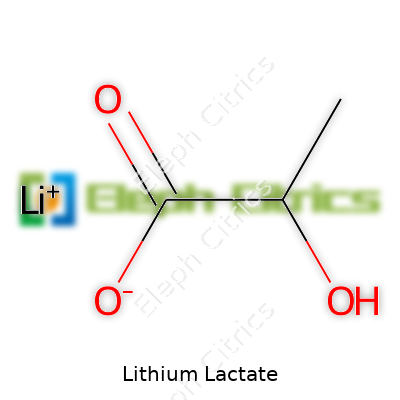
Lithium lactate sits in a class of lithium compounds gaining attention for their unique combination of solubility and bioactivity. Chemically, it represents the lithium salt produced from lactic acid, carrying the molecular formula C3H5LiO3. Markets offer lithium lactate in several forms, most often as a white, fine powder or a clear liquid. Each production batch targets high purity, especially for pharmaceutical or analytical applications, because trace metals and organic byproducts risk interfering with sensitive end-uses. Pharmacies, laboratories, and even food chemistry labs reach for lithium lactate due to its blend of mild alkalinity and organic compatibility.
Lithium lactate spreads easily and dissolves swiftly in water, echoing other lithium salts in its basic handling. The powder form appears white with no strong odor, and forms a nearly neutral-to-mildly alkaline solution in water. It resists decomposition at ordinary temperatures, which helps with safe handling and storage. Compared to other lithium salts, lithium lactate holds the advantage of mild reactivity, neither explosive nor prone to hazardous gas release under simple heating. Chemists appreciate its molecular weight, about 96.02 g/mol, and its pleasant predictability under standard lab conditions.
Industry buyers scrutinize certificate of analysis sheets for lithium lactate. Typical technical specifications demand at least 98% assay on a dry basis, with water content usually trimmed below 2%, and limits on heavy metals far beneath the safety threshold. Containers carry clear labeling, marking the product’s chemical name, formula, batch number, potential hazards, and recommended storage. Any hazard statements—usually aimed at handling lithium ions—come printed in visible fonts, not buried in fine print, providing crucial information for safe laboratory or industrial use. Some batches carry designations around their suitability for pharmaceutical, analytical, or food-intermediate status.
Production teams combine lactic acid with lithium carbonate or lithium hydroxide under carefully controlled conditions, stirring the acids and bases in reactor vessels until complete neutralization occurs. The solution gets filtered to remove any unreacted starting materials or insoluble impurities. Manufacturers can then concentrate and dry the solution, collecting the crystalline solids or further purifying to offer it as a liquid concentrate. Quality-control checkpoints along the way use chromatography and titration to confirm low residual lithium salts or contaminants, since buyers run their own tests and reject off-spec shipments outright.
Lithium lactate reacts readily with strong acids, swapping lithium for another cation through double displacement, a classic staple of elementary chemistry classes. In presence of strong bases, it can break down further, but typically remains stable in neutral and mildly alkaline environments. Chemists sometimes modify lithium lactate for specialty applications, attaching various functional groups to the lactate backbone, broadening the range for advanced synthesis or targeted medicinal purposes. Research teams look at how to tweak the molecule for specific tasks—creating new reagents or intermediates with slightly shifted solubility profiles or catalytic characteristics.
Across technical literature and supply catalogs, lithium lactate turns up under names like Lithium 2-hydroxypropanoate and Lithium alpha-hydroxypropionate. Sometimes suppliers feature minimalism, sticking with the simple “lithium lactate,” while others brand it with trade-specific codes or references. Old chemistry books occasionally refer to it as lithium salt of lactic acid, causing a brief dance with confusion for anyone cross-referencing different eras of chemical records. Most researchers or industry buyers stick with “lithium lactate” when searching for performance data, toxicity reports, or stock availability.
Safety data sheets for lithium lactate draw a middle ground: not as hazardous as lithium hydride, but still deserving respect. Workers wear gloves and sometimes goggles, protecting from mild skin or eye irritation. Inhalation, while unlikely given its low volatility, should be avoided, and spills cleaned up using standard lab absorbents. Storage rules call for cool, dry places away from acids or foods. Any medical or pharmaceutical labs handling this salt train staff to handle lithium solutions carefully, given lithium ion’s impact on cardiac and neurological function when ingested chronically or at high doses. Disposal guidelines go through regular review to align with local environmental protections, ensuring low risk of lithium contamination in water or waste streams.
Chemists bring lithium lactate into pharmaceutical research because it acts as a gentle lithium ion donor, supporting studies on mood stabilization and neurochemical modulation. Nutrition-focused labs explore its bioavailability, seeking alternatives to sodium-based lactates in food preservation or sports supplementation. Analytical labs measure how lithium lactate helps as a buffer or reagent, especially where mild alkalinity steadies tricky reactions. Industrial users experiment with it in polymerization reactions, taking advantage of the lithium ion’s unique effect on reaction kinetics. Health research circles keep finding new biochemical pathways where lithium lactate provides insights, driving the expansion of its application portfolio.
Companies and university labs keep exploring lithium lactate’s roles that cross from health sciences to materials innovation. Lithium’s effect on neural activity continues to prompt deep-dive research into new treatment approaches for mood disorders, bipolar depression, and neurodegenerative conditions. R&D teams compare lithium lactate with other lithium compounds, hoping to reduce side effects or boost effectiveness by leveraging lactate’s natural involvement in human metabolism. Material scientists dig into how lithium lactate influences rubber curing, polymer chain length, and other industrial details no less important than lab successes. Such research leans heavily on robust empirical results, where small shifts in impurity or formulation swing the final results.
Scientists pay close attention to lithium lactate’s safety record, since lithium in high doses poses risks to renal, cardiac, and nervous systems. Animal studies show moderate acute toxicity at doses much higher than typical medical exposures, but even modest overdoses can trigger warning signals in humans. Researchers focus on cumulative exposure, chronic consumption, and how patients metabolize and excrete lithium delivered as lactate compared to carbonate or citrate salts. Reports also address environmental risks, knowing that lithium ions disrupt aquatic bioactivity if discharged unchecked. Public databases compile toxicity scores from rodent studies and long-term metabolic experiments, driving modern safety protocols and daily dose limits in all lithium-based products.
Demand for lithium lactate is climbing as pharmaceutical researchers look for gentle, bioavailable lithium salts that sidestep the complications found with older compounds. Battery and materials scientists eye any lithium carrier that offers cleaner, safer, or easier handling for niche manufacturing techniques. Medical research has started considering lithium lactate as a possible entry point for new therapies in brain health and metabolic disease, given its roots in natural metabolic cycles. Environmental scientists investigate biodegradable lithium salts as safer alternatives in water treatment and renewable energy storage. Progress on toxicity and long-term metabolic pathways will anchor whether lithium lactate stays on its current upward path, shifting from a research staple to a widely adopted component in the next generation of advanced medical and industrial products.
Lithium lactate doesn’t get the same buzz as lithium-ion batteries or even lithium carbonate, but its name comes up in some quiet corners of science and medicine. This compound, a salt formed by combining lithium and lactic acid, looks pretty plain on the surface. Like many lithium salts, people mostly know about its calming effects in mental health treatments, but lithium lactate brings its own history and uses.
Doctors once prescribed lithium salts for a range of mental health problems. Lithium carbonate still shows up as a frontline mood stabilizer for people with bipolar disorder. Lithium lactate traveled along a similar path. Doctors believed it could help tamp down racing thoughts and bring relief to certain kinds of mania. Some early 20th-century medical guides suggested lithium lactate for nervousness and agitation, especially before more modern medicines arrived.
Lithium lactate dissolves easily in water, making it simple for doctors to mix into liquid medicines. That made it easier to give in smaller doses or to children who struggled with tablets. Even so, concerns grew over its side effects, since lithium has a narrow safety margin. Too much can stress the kidneys or heart, and regular blood tests became a must. Over time, more precise and predictable forms like lithium carbonate or citrate replaced lithium lactate for most psychiatric uses.
Labs continue digging into lithium lactate. Researchers study it not just for its mood-stabilizing effects, but for how it influences cells. Lithium salts impact certain cellular switches known as enzymes, especially ones that play a role in how brain cells send messages. There’s growing interest in how these effects might protect nerve cells from damage, opening possible doors for conditions like neurodegenerative diseases.
Some teams experiment with lithium lactate because it dissolves easily, helping mix it into certain types of tissue studies. By using it in cell cultures, scientists hope to sort out which lithium compounds work best and which might cause fewer side effects.
Despite some historical medical uses, doctors rarely prescribe lithium lactate in the United States today. The Food and Drug Administration (FDA) never approved it for regular psychiatric treatment. Most clinicians rely on lithium carbonate or citrate, which have undergone decades of scrutiny and tweaking to balance effect and risk.
It’s tough to talk about any lithium compound without touching on side effects. Lithium salts, lactate included, can throw off sodium and fluid balance, stir up nausea, and strain the thyroid and kidneys. No over-the-counter version gives safe benefits without close supervision. Blood checks and experienced guidance make a difference for people who benefit from lithium’s stabilizing pull.
Although its direct use in medicine has faded, lithium lactate sometimes appears in specialized biochemical labs and niche industrial setups. For example, it occasionally shows up in certain chemical synthesis or as a buffer in laboratory processes. Its solubility lets researchers create stable solutions for experiments. Real talk, most people will never bump into it outside of research buildings.
Most attention and investment today surround the use of lithium in renewable energy and classic psychiatry. If lithium lactate earns a spot back in the mainstream, it will depend on whether future studies uncover unique advantages or fewer problems than other lithium-based medicines. For now, it serves as another reminder that even old, almost-forgotten salts can still shape what happens in science.
Lithium lactate doesn’t come up in conversation very often, but it’s found its place in medicine for managing mental health concerns like bipolar disorder. The goal has always been to steady those intense mood swings, making daily life a little more manageable. Treatments that involve lithium aren’t new. The compound shows up on prescription lists because science backs its ability to calm the extremes. The flip side, though, brings its own set of challenges, mostly centered on side effects that can complicate things.
Most people who try lithium-based medications notice something happening. Dry mouth often starts early, leading to a constant hunt for water. Thirst feels stronger than usual, even without breaking a sweat. Nausea can join the mix, which throws off regular eating routines. A shaky feeling in the hands–the so-called “lithium tremor”–makes simple things like holding a fork a trickier task. Some people talk about tiredness that never loosens its grip, and with attention slipping, work and study suffer. Weight gain isn’t rare either, which feels unfair for those already handling heavy emotional shifts.
While nobody enjoys an upset stomach, the real red flags pop up with kidney and thyroid function. The kidneys play a big part in clearing lithium from the body. Over time or with too much in the system, problems show as frequent urination, changes in blood pressure, or new headaches. Blood tests reveal these issues before most symptoms surface, but not everyone gets checked as often as needed. Thyroid issues might sneak up too, taking the form of unexplained weight shifts or a constant chilled feeling, even under blankets. A 2021 meta-analysis published in Frontiers in Psychiatry confirmed that regular monitoring of thyroid and kidney values helps lower the risk of missed complications.
High lithium levels in the blood call for urgent attention. Signs like slurred speech, muscle weakness, blurred vision, or confusion can show that too much lithium has entered the bloodstream. Doctors call this lithium toxicity. If ignored, it can damage organs and threaten life. Older adults and people with heart problems face the highest risk. Keeping salt and fluids steady reduces this danger, so skipping regular lab tests isn’t wise. Reports from the US Food and Drug Administration have shown that accidental overdoses have sent patients to the hospital if warning signs were shrugged off.
No one wants to add more trouble to their plate. So, it matters to set up blood tests before starting lithium lactate and adjust doses based on the results. If side effects creep in, flagging them early with a doctor makes a difference. Hydration helps, especially during hot weather or illness, with steady salt intake playing a quiet but crucial role. Doctors sometimes adjust the medicine, change brands, or combine treatments to ease the harder side effects. Paying attention to physical signals–anything new like strange thirst, tremors, or confusion–gives enough notice to get help before serious issues grow.
Treating mood disorders never feels simple, but transparent conversations about the risks bring power back to patients. Family members who know the signs of trouble can support their loved ones, nudging for help if something seems off. By shining a light on possible side effects and speaking up about them, it’s possible to get the benefits of lithium lactate without the worst surprises. Safety comes down to listening to your body and asking for answers as soon as things seem unfamiliar.
Anyone looking for direction on lithium lactate will find more questions than answers in public forums. Most folks at home have never even seen it in a prescription bottle, and for good reason. The compound never earned the kind of trust lithium carbonate sits on for mood stabilization. Some supplement sellers still toss it into the mix, pushing its mineral side or nodding to possible effects, but the evidence just isn’t lining up. Let’s dig into how people have actually used it, why professionals are cautious, and where real-life experience pulls its weight.
Lithium treatment, even as carbonate or citrate, has always meant walking a tightrope. Too little, and you don’t get the benefits. Too much, and the kidneys raise the alarm—tremors, confusion, muscle twitches, maybe much worse. Unlike sodium, lithium isn’t found in processed foods or everyday shakes, so small missteps stack up fast. Human kidneys work hard to clear it, but they don’t always manage. In my own experience discussing these issues with pharmacists, they double-check scripts and blood histories every time, because toxicity can show up by surprise even after months on the same dose.
Outside the supplement world, published dose ranges for lithium lactate just don’t exist. Universities dug into its chemistry a century ago, but those studies faded away after lithium carbonate took over in psychiatry. Anyone who remembers the old Merck works of the 1940s knows the doses were always in terms of “elemental lithium,” not the salt. Today, clinical guidelines barely mention lactate. Even the FDA doesn’t list lithium lactate on its standard drug databases. That says something about confidence in safe, effective dosing.
Researchers tie lithium’s effects directly to blood level, no matter if it’s carbonate or some rare salt. Typical maintenance therapy aims for 0.6 to 1.2 mEq/L in serum; going above brings side effects and hospital visits. When generalists hand out lithium for nutrition or off-label reasons, there’s usually no blood testing or medical safety net. Unlike magnesium or potassium, nobody should guess at the right level. Just one week's misdose can stretch a heart rhythm, drag down energy, or even trigger seizures.
People interested in mood health or trace minerals deserve honest guidance. For those needing lithium, only a medical provider should set dosing, with regular bloodwork. Folks already on psychiatric medication will notice nurse calls when checks are overdue—it’s not bureaucracy, it’s life-saving practice. Pharmacists can translate between different salts using conversion charts, but always with the base plan set by physicians. There’s no magic pill hiding in the supplement aisle. If a product promises to fill gaps or change moods, it needs to show safety and proof. Until lithium lactate gets FDA attention or better research, using it without supervision runs real risk.
Trust builds through checked labs, open communication with doctors or pharmacists, and honest talk about side effects. Anyone considering lithium—any salt—ought to know the risks long before picking up a bottle, and should demand that kind of care from every health provider.
Lithium has saved lives for people facing severe mood swings. In the right hands, it brings stability. Most folks know lithium carbonate tablets, but lithium lactate comes up sometimes—especially in compounding or alternative formulations. Like any form of lithium, it creates big concerns if mixed with other medications without careful management.
Doctors keep close tabs on lithium levels for good reason. Even slight changes in dosage, diet, hydration, or other drugs can tip someone into lithium toxicity or lower it enough that benefits disappear. Lithium lactate breaks down in the body, adding both lithium ions and lactate to the bloodstream. Lactate itself isn’t usually the troublemaker, but everything that increases lithium levels or stops the body from flushing it out can spell real danger.
Blood pressure medications called diuretics pull fluid and sodium from the body. Kids with a science set might remember that sodium and lithium like to get filtered out of the body in the same way. Lose too much sodium, and lithium sticks around, climbing into the toxic range. Thiazide diuretics have landed many people with lithium toxicity in the ER—even more so if they don’t drink enough water.
ACE inhibitors and angiotensin receptor blockers, popular for heart or blood pressure problems, raise the risk as well. They change kidney function, slowing down how fast the kidneys get rid of lithium.
Ibuprofen, naproxen, and other over-the-counter drugs are everywhere. People pop them for pain and headaches, then forget their kidneys get stressed. These painkillers slow down kidney blood flow. If there’s lithium in the system, the pressure change means it doesn’t get cleared efficiently. Creatinine creeps up, lithium follows, and the typical signs—nausea, shakes, confusion—start to show. I’ve seen patients who never imagined a pain reliever could put them at risk, but it happens.
Often, folks with bipolar disorder or depression take lithium lactate alongside SSRIs, SNRIs, or antipsychotics. Mixing these can sometimes trigger a problem called serotonin syndrome or neuroleptic malignant syndrome. It’s rare, but adding lithium into a cocktail of psychiatric meds means every new symptom matters. I once knew a patient who suddenly got muscle stiffness and a fever after her doctor added a new antipsychotic to her mix—she ended up in the hospital, and it wasn’t easy to sort out.
Believe it or not, coffee or energy drinks matter, too. Caffeine ramps up urination and may drop lithium levels unexpectedly. Then, add in a weekend of sweating outside or cutting out table salt, and the whole balancing act can go off track.
Alcohol, dehydration, and even plain fever turn the equation upside down. Any doctor who works with lithium runs blood tests often. That’s not overkill; it saves lives.
Honest conversations with pharmacists and doctors matter. Patients on lithium lactate should keep everyone in the loop—no secrets about supplements, new pills, or even sudden diet changes. Some pharmacies use software that flags these interactions automatically, but personal oversight works best.
Keeping written lists, reporting every new drug (even herbal teas), checking blood work, and staying hydrated all help. And no medication changes without a real plan.
Hard lessons usually teach better than any pamphlet. Families who’ve dealt with lithium complications know that drug interactions turn up fast and don’t always give a warning. Careful management and open eyes stay more useful than any warning label.
Lithium lactate is a salt form of lithium, often found in supplements and sometimes in alternative medicine products. It doesn’t share the same legal status or scientific backing as prescription lithium carbonate, which doctors prescribe for bipolar disorder and some other psychiatric issues. Many people see "lithium" on a label and think only of mood stabilizers, but these products differ. While prescription lithium has been studied in pregnant and breastfeeding women, over-the-counter forms like lithium lactate lack robust research on safety, especially for anyone expecting a child or nursing.
Doctors use prescription-strength lithium cautiously among pregnant patients. Decades of research show lithium can help control severe mental illness, but it comes with risks. Lithium crosses the placenta easily, reaching the developing fetus at levels close to those in a mother’s blood. Big studies dating back to the 1970s linked lithium use during pregnancy, particularly during the first trimester, with an increased risk of heart defects in babies, especially a rare defect called Ebstein’s anomaly. Modern research suggests the risk exists but may be lower than people first thought, affecting about 1 in 1,000 exposed infants.
The risks with lithium lactate—used outside of a doctor’s careful monitoring—are less clear-cut, since doses in supplements vary or go unlisted. While lithium orotate and lactate are sometimes marketed as safer “natural” alternatives to pharmaceutical lithium, the human body processes all lithium forms in a similar way after absorption. Researchers haven’t proven that these forms dodge the health risks of prescription-strength compounds.
Lithium appears in breast milk if a mother takes any form of lithium. Studies following babies exposed through breast milk show lithium can build up in infant blood to significant levels, more so in newborns whose kidneys haven’t developed the ability to flush it out. Some babies become restless and show poor feeding. Others don’t seem to react, but few long-term studies check for subtle or delayed problems. Top medical authorities like the American Academy of Pediatrics and World Health Organization advise mothers to avoid lithium while nursing, or to use it under strict medical guidance with close infant monitoring.
Doctors don’t recommend lithium lactate or related products for people who are pregnant or breastfeeding because they haven’t seen enough proof they’re safe. Adding extra lithium, even from a supplement, could push blood levels high enough to cause side effects in both the parent and the baby. Risks can sneak up because supplement labels often skip listing exact doses, or batch-to-batch variation creates inconsistent exposure.
If struggling with symptoms of depression, mood swings, or other mental health problems while pregnant or nursing, reaching out to a qualified healthcare provider makes a difference. Safer, evidence-based treatments are available, and medical professionals can weigh the risks and benefits based on individual needs.
Instead of reaching for an over-the-counter product, checking in with a doctor ensures both mother and child stay as safe and healthy as possible. Keeping open lines of communication with care teams builds trust, catches issues early, and supports the wellbeing of both baby and parent during these crucial times.
| Names | |
| Preferred IUPAC name | lithium 2-hydroxypropanoate |
| Other names |
Lactic acid lithium salt Lithium 2-hydroxypropanoate |
| Pronunciation | /ˈlɪθiəm ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | [867-56-1] |
| Beilstein Reference | 390422 |
| ChEBI | CHEBI:64681 |
| ChEMBL | CHEMBL1201607 |
| ChemSpider | 92326 |
| DrugBank | DB09407 |
| ECHA InfoCard | 04dd99ba-7b8e-4d48-b75d-bbaf1d918774 |
| EC Number | 208-058-8 |
| Gmelin Reference | 5794 |
| KEGG | C18683 |
| MeSH | D008091 |
| PubChem CID | 23668537 |
| RTECS number | OD9625000 |
| UNII | J2K21F2LK6 |
| UN number | UN3265 |
| CompTox Dashboard (EPA) | DTXSID7020183 |
| Properties | |
| Chemical formula | C3H5LiO3 |
| Molar mass | 128.06 g/mol |
| Appearance | White crystalline powder |
| Odor | odorless |
| Density | 1.20 g/cm3 |
| Solubility in water | Soluble |
| log P | -0.64 |
| Acidity (pKa) | 3.67 |
| Basicity (pKb) | 11.77 |
| Magnetic susceptibility (χ) | −37.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.423 |
| Viscosity | Viscosity: 5.27 cP |
| Dipole moment | 3.07 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 166.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -789.3 kJ/mol |
| Pharmacology | |
| ATC code | N05AN01 |
| Hazards | |
| GHS labelling | **GHS labelling**: `"Warning; H315, H319, H335; P261, P305+P351+P338"` |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: H302, H319 |
| Precautionary statements | H302: Harmful if swallowed. P264: Wash hands thoroughly after handling. P270: Do not eat, drink or smoke when using this product. P301+P312: IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell. P330: Rinse mouth. |
| NFPA 704 (fire diamond) | 1-1-1 |
| Lethal dose or concentration | LD50 Oral Rat 354 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 525 mg/kg |
| NIOSH | SN3850000 |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 2-5 g |
| Related compounds | |
| Related compounds |
Sodium lactate Potassium lactate Calcium lactate Magnesium lactate |