People have leaned on magnesium carbonate for centuries, far longer than most realize. Ancient Greeks and Romans made use of magnesite stones, scratching them onto teeth and using them as fireproofing. By the late 1700s, chemists began isolating and studying this mineral, fascinated by its resistance to heat. In the 20th century, magnesium carbonate took on a new presence in factories and sports arenas. Manufacturers found ways to extract it on an industrial scale, paving the way for advances in ceramics, pharmaceuticals, and even climbing. Its connection to chalk—used on athletes’ hands and billiard tables—goes back to those early studies, and many of the applications we rely on today grew from simple, practical observations made two hundred years ago.
If you’ve ever gripped a gym bar or watched a gymnast at work, you’ve seen magnesium carbonate in action. The material stands out for its ability to absorb moisture, which explains its starring role as “chalk” for athletes. Powdered or granulated, it appears as a fluffy white solid—pretty bland at first glance, but incredibly useful. Its basic chemical formula, MgCO3, keeps it stable under most everyday conditions. This mineral goes into antacids, laxatives, food supplements, rubber products, paints, and more. Long before it reached medicine bottles or climbing gyms, it started as raw magnesite ore, dug from hillsides and riverbeds worldwide, from China to Slovakia.
Magnesium carbonate kicks off with a melting point well above many common compounds—linear heating only breaks it down above 350°C. It doesn’t dissolve easily in water; in fact, barely at all. In my experience working in labs, this fact makes it a frustrating impurity when you’re aiming for soluble salts. The powder looks soft and light, but feels rough and dry. The density averages around 2.95 g/cm3. As a base, it reacts gently with acids, fizzing and releasing carbon dioxide. Pure samples lack any strong smell or taste, which helps when adding it to foods and pharmaceuticals. In the right environment (humid air, for example), it can slowly soak up carbon dioxide, so manufacturers package it carefully to avoid unwanted moisture.
Factories offer magnesium carbonate based on strict standards. Purity levels sort it into technical, food, or pharmaceutical grades. Color often signals quality—whiter products usually pass higher-grade tests. Impurities like iron, chloride, and heavy metals face tight limits, according to groups such as the USP or EINECS. Accurate labeling spells out composition, net content, batch number, the manufacturer’s address, and any special handling warnings. If you’ve bought supplements online or at a pharmacy, you’ll see details about particle size, moisture content, trace element testing, and expiration dates—all of which track back to global food and drug safety rules. Safety data sheets offer guidance on disposal and accident handling, keeping both workers and the public safe.
Companies usually start by extracting magnesite or dolomite rock. After crushing and cleaning, acids or carbon dioxide gas react with magnesium-rich brines or ore. The result is a slurry that settles out as magnesium carbonate. The process needs careful control—if the pH drifts, impurities creep in. After filtration and washing, drying turns the wet cake into a pure, storable powder. Some plants use high pressure and temperature to tweak the product’s properties for special uses. Chemical engineers adjust every step to boost yield and cut waste, showing the intersection of old-world mining skills and today’s green chemistry. Even minor technical missteps, like traces of silica, can cause quality headaches down the line, so attention at each stage matters.
Magnesium carbonate finds itself at the center of both practical and experimental reactions. Adding any strong acid will quickly produce carbon dioxide gas and a magnesium salt—hydrochloric acid, for instance, yields magnesium chloride and water. Magnesium carbonate can convert into magnesium oxide by simple heating, a process common in refractory ceramics and catalyst manufacturing. Tinkering with process conditions lets chemists make basic magnesium carbonate, hydromagnesite, or artinite, each with its quirks and uses. Blending or doping magnesium carbonate with other compounds changes its reactivity for specific chemical processes, like environmental cleanups that use it to soak up heavy metals from water. Over my years in chemical research, I’ve seen surprising innovations—surface treatments improve water dispersibility, and tiny crystal adjustments give drugmakers better excipient control.
You’ll run into several aliases in both literature and on product labels. Heavy magnesium carbonate, light magnesium carbonate, magnesite, and hydrated forms like hydromagnesite all show up. For athletes and scientists, it’s just “chalk.” Drugstores use names like magnesium salt, carbonic acid magnesium salt, or magnesium carbonate, natural or synthetic. CAS Number 546-93-0 pins it down for regulators. In trade catalogs, you’ll spot “mag carb,” “precipitated magnesium carbonate,” or industry codes tied to purity levels. Trying to order chemicals without knowing these synonyms usually ends in confusion and wasted time. Accuracy in naming keeps purchases and handling straightforward, ensuring folks get exactly what they need.
Handling magnesium carbonate rarely sets off alarms, but safety always matters. Inhaled dust can tickle the nose and throat. Repeated or high-level exposure, especially in a dusty workplace, has been known to irritate lungs over time. Safety regulators, like OSHA or the European Chemicals Agency, lay out exposure guidelines and recommend common sense—good ventilation, gloves if contact is frequent, and masks during heavy use. Food-grade material faces even tighter monitoring, preventing microbe contamination or heavy metal build-up. Storage means keeping containers dry and away from acid fumes, since even routine leaks can set off unwanted chemical reactions. Factories audit their operations, track accidents, and train workers to spot trouble before it snowballs.
Magnesium carbonate works its way into daily life more than folks realize. The food industry uses it as an anticaking agent—think powdered soups, table salt, and spice mixes where clumping would be a pain. Pharmaceutical companies rely on it as a filler in tablets, helping medications break apart smoothly. Sports professionals chalk their hands to boost grip, while climbers and weightlifters trust it for sweat absorption and slip prevention. Beyond athletics, ceramic manufacturers use it to increase strength in tiles and bricks. In dentistry, it appears in products from polishing powders to gentle abrasives. In my own kitchen, I’ve even used it during recipe testing to keep spices flowing. Environmental projects harness its ability to neutralize acids in water or soil, making it valuable for remediation efforts.
Scientists keep looking for more out of magnesium carbonate. Lab efforts target better particle control for uses in drug delivery, where shape and size affect how medicine dissolves and works in the body. Materials engineers tweak its properties so it works longer as a flame retardant. In agriculture, trials track its ability to ease magnesium deficiency in crops—especially on soils that resist common fertilizers. Cutting-edge researchers study how it locks away carbon dioxide, hinting at climate fixes through mineralization. Over time, even well-known materials benefit from fresh eyes, and funded research in Europe, China, and North America aims for breakthroughs in purification, nano-sized particles, and industrial recycling. Collaboration between universities and companies brings new ideas forward, from cheaper manufacturing to entirely new applications.
Magnesium carbonate’s safety record holds up well compared to many industrial additives. Decades of animal testing and human case studies point to low toxicity. Swallowed in large amounts, it can upset digestion, but most people only encounter it in tiny doses, far below thresholds linked to health concerns. The compound passes swiftly through healthy kidneys, causing no accumulative danger. Rare allergic reactions pop up on occasion, though, which highlights why the food and drug industry tracks surveillance data and investigates consumer complaints. Regulatory reviews update limits as new information surfaces. I’ve reviewed case data on factory workforces and rarely found evidence of chronic illness. Environmental impacts also seem minimal, since the mineral form breaks down slowly and doesn’t poison soil or groundwater.
Magnesium carbonate won’t fade away anytime soon. Its use in green technologies looks set to rise, driven by needs in CO2 capture, environmentally benign construction, and low-dust sporting goods. With stricter rules on food and drug safety, manufacturers invest more in purity, traceability, and smart packaging. As new uses take shape—like magnesium-based batteries or advanced ceramics—producers tweak synthesis methods and properties to serve high-tech industries. Few substances switch so easily between kitchen, pharmacy, gymnasium, and laboratory, making magnesium carbonate both adaptable and dependable. Scientists and engineers have only scratched the surface of its abilities, and future advances may open the door to even safer, cleaner, and more efficient ways to use this old but valuable mineral.
Magnesium carbonate shows up in daily life far more than people realize. In high school, rock climbers and gymnasts taped up and dusted their hands with white chalk for a better grip. Turns out, that “chalk” was almost always magnesium carbonate. It absorbs sweat fast, cutting down on slipping. Walk into a climbing gym or weight room, and you'll see white powder handprints everywhere—an unspoken sign people value safe, strong holds.
This same powder makes appearances in food and medicine too. As a supplement, magnesium carbonate helps people who need more magnesium in their diets. Many don’t absorb enough magnesium from regular meals, which leads to muscle cramps or tiredness. Swallowing a few tablets can top up those mineral levels. Pharmacists recommend supplements like these not just for athletes, but also for folks with certain health issues or those following strict diets.
Food manufacturers turn to magnesium carbonate as an anti-caking agent. Recipes for table salt, spices, and even cocoa powder all run into problems when moisture creeps in and clumps ingredients together. Adding a dusting of magnesium carbonate keeps powders loose, so people get a smooth pour every time. Safety agencies like the World Health Organization reviewed these uses and marked magnesium carbonate as safe in small amounts, so no need for worry with that morning coffee or dinner seasoning.
Long talks with pharmacists taught me that magnesium carbonate isn’t only about nutrition. In antacid tablets, magnesium carbonate soothes heartburn and eases indigestion. Hospitals stock these chewable tablets for quick relief. Magnesium carbonate neutralizes stomach acid, providing a gentle option for people who deal with sensitive stomachs. Because it draws water into the intestines, doctors sometimes use it as a mild laxative, which helps patients with constipation.
Magnesium carbonate tackles cleaning tasks on an industrial scale. Workers in steel plants dust their hands with it during hot, slippery jobs. It also plays a part in making rubber and plastic products last longer, so companies use it to bulk up materials without weighing them down. Environmental engineers see potential for magnesium carbonate in tackling carbon emissions. When trapped with CO2, magnesium carbonate forms solid byproducts that don’t leak back into the air. Scientists keep studying this reaction as a possible climate solution.
Most of us don’t think about what happens after eating a meal with magnesium carbonate added as an anti-caking agent. Scientists at the University of California reviewed the ingredient and found that, used properly, it won’t upset healthy digestive tracts. People with magnesium sensitivities or kidney conditions should talk to their doctor before adding supplementation. In my own experience with iron supplements years ago, trying other minerals like magnesium needed simple, honest chats with my doctor—good advice for anyone considering them.
Easy access to magnesium carbonate lets people manage everything from grip in sports to mild digestive upset. Companies carry a responsibility to update product labels and not quietly sneak in unnecessary fillers. Consumers should read ingredient lists and talk to professionals about their own health needs. By keeping an eye on how it’s used, the benefits of magnesium carbonate can outweigh any confusion or concern. Smart, informed choices matter. If you’re thinking about supplements or want the best grip on the wall, knowing magnesium carbonate’s real roles pays off in everyday life.
You’ve probably seen magnesium carbonate listed on a food label or as a supplement ingredient. Known for keeping table salt from clumping, it finds its way into many processed and packaged foods. Some folks notice it in their daily multivitamin as well. Those little white chalky tablets in climbing gyms or under the hands of weightlifters — that’s also magnesium carbonate. Different purpose, of course, but all the same stuff.
Magnesium pops up in green vegetables, nuts, and whole grains. Our bodies lean on magnesium for nerve function, muscle movement, and bone strength. For folks falling short, supplements can fill the gap. Magnesium carbonate itself isn’t the form our bodies use most easily, but in the stomach's acidic environment, the compound converts to magnesium chloride, making its magnesium available for absorption.
If you stick to the recommended dietary dose, science finds magnesium carbonate safe to eat. The U.S. Food and Drug Administration gives it a green light as a food additive. The European Food Safety Authority also places it squarely on their list of safe food ingredients. For most healthy adults, the magnesium intake limit lands at about 350 milligrams per day from supplements. Getting more than that from food rarely causes trouble, since the kidneys handle the extra.
Doctors and dietitians use magnesium salts like magnesium carbonate to help with heartburn or stomach acid. These over-the-counter antacids don’t just settle the stomach, they also top up magnesium for anyone who needs it. Out of habit, those with chronic kidney issues, or very high supplement intake, want to watch magnesium—including its carbonate form—since the body might not clear out the surplus as easily. Too much magnesium can bring on diarrhea, cramps, or in rare cases, heart problems.
Folks grabbing handfuls of supplements without tracking the total dose sometimes run into side effects. Diarrhea ranks as the top complaint, since magnesium draws water into the gut. If you ever mixed up a too-concentrated glass of magnesium, you probably know. Certain medicines—like diuretics or some heart drugs—change the way the body holds on to minerals, including magnesium. Taking large doses along with these meds should call for a doctor’s advice.
Powdered magnesium carbonate used for athletic grips is not the same as food-grade; breathing the dust or swallowing accidental bits probably won’t hurt a healthy person, but it isn’t made for eating.
Most folks do fine just checking nutrition labels on supplements and foods, and sticking with established brands. For people on medication or with kidney conditions, talking through supplement plans with a doctor helps avoid complications. Doctors can run simple blood tests if anyone worries about mineral levels. If you ever feel stomach discomfort after a supplement, cutting back usually solves the problem. Eating a balanced diet remains the best route toward steady magnesium intake.
Keeping magnesium in the safe lane mostly means watching the amount, not skipping it altogether. Like most minerals, balance makes the difference between healthy support and unwanted side effects.
Magnesium carbonate often finds its way into supplements and some over-the-counter antacids. Plenty of athletes reach for it too, sometimes in powdered form to reduce moisture and sweat on hands for a better grip, especially in sports like rock climbing or gymnastics. As a supplement, the goal is to help boost magnesium levels, a mineral that plays a key role in over 300 enzyme systems in the body. But reaching for extra magnesium doesn’t come without possible bumps along the road.
A lot of people notice stomach discomfort or a laxative effect if they go heavy on magnesium carbonate. This isn’t surprising; this compound doesn’t get fully absorbed, so what your body can’t take in, it sends to your digestive tract—often leading to diarrhea or loose stools. That side effect often feels uncomfortable, disrupting daily life for anyone not used to it. People with sensitive stomachs end up with cramps, bloating, or nausea. Medical experts say this uptick in bowel activity can go from mild to troublesome depending on the dose or individual sensitivity. Having worked with endurance athletes, I’ve seen a few regretful faces after taking unfamiliar supplements on race day, wishing they’d tested their gut at home instead. It can be a gamble without knowing your own limit.
Supplementing with magnesium carbonate goes beyond just digestive discomfort. If taken in large amounts, excess magnesium can build up, especially in people with kidney issues. Healthy kidneys usually get rid of what isn’t needed, but if they’re not working well, magnesium can collect in the blood. Too much in the system may lead to symptoms like unstable heart rhythms, low blood pressure, or muscle weakness. Severe cases may trigger confusion, trouble breathing, and even cardiac arrest; though rare, the risk grows if someone combines magnesium supplements with antacids or laxatives, or isn’t aware of their own medical vulnerabilities. According to scientific research, adults shouldn’t get more than around 350 mg per day from magnesium supplements, unless a doctor says otherwise.
On occasion, magnesium carbonate can also spark allergic reactions. That sounds unlikely, but hives, rashes, or difficulty breathing are medical emergencies demanding attention. I’ve never seen this first-hand, but even rare events matter when the stakes are high. People already sensitive to food additives or drugs need to double check every label and talk with a doctor before introducing anything new.
Those with kidney disease, heart conditions, or certain gastrointestinal disorders should take magnesium carbonate only under medical guidance. Medications for heart rhythm and blood pressure management can interact with magnesium, altering how both work. If you take diuretics, antibiotics, or drugs for osteoporosis, consult someone who knows your full medical picture. It’s easy to think of minerals as benign, but even low-risk supplements aren’t risk-free.
Sticking with food sources like leafy greens, beans, and nuts is a safer bet for hitting magnesium needs. If you use supplements, start with low doses and pay close attention to how your body reacts. Report unusual symptoms to a healthcare provider. The FDA doesn’t regulate supplements closely, so look for third-party certifications on products to reduce the odds of contamination or mislabeling. Reliable brands publish batch test results for peace of mind.
Walk into any lab or supply room and you’ll spot containers of magnesium carbonate on the shelves. This powder turns up almost everywhere—gyms, classrooms, tablet presses, climbing bags. People tend to treat it like baking soda, not thinking much beyond the white powder. Trouble starts when humidity finds its way into the jar. Magnesium carbonate absorbs moisture like a sponge, clumping fast and losing its usefulness. Damp conditions can even trigger chemical changes if other reactive substances are nearby. That clump in the corner is more than an inconvenience for athletes or chemists; it opens the door to wasted batches or flawed results.
Years of working with chemicals drills a few habits into anyone’s routine. Keep powders dry, separate, and sealed. For magnesium carbonate, these rules make a real difference. Water hides everywhere—in sweaty gym bags, on lab benches, in steamy storerooms. Even a cracked lid lets the air sneak in and ruin the product. Humidity might seem like a small risk, but that extra moisture allows the powder to become a brick or, worse, interact with acids or other chemicals in the same cabinet. You can spot who learned the hard way from the folks who keep a tight lid and label everything clearly.
Plastic screw-top jars with a good seal block most air and moisture. Glass works just as well if you keep hands dry and the lid tight. Big sacks for industrial labs get double-sealed, with an extra plastic liner inside the main bag. Chalk for climbing and gymnastics comes pre-packed in foil bags sometimes for the same reason—extra protection from a damp day. I’ve seen metal tins rust from being kept in a damp locker, and that rust can work its way in. Shelves well above the floor help, too; you avoid leaks or spills that start low and work their way up. Chemicals packed low on warehouse floors always end up in the path of a mop or a puddle.
Keeping magnesium carbonate with acids or with materials that react to moisture is asking for problems. Strong-smelling solvents, acid bottles, and oxidizers each deserve their own space. Cross-contamination rarely starts with a spill; more often it comes as dust or vapor floating between bottles left open on a bench. Separate shelves for inorganics and acids cut down on those risks. Some workplaces use dedicated lockers with ventilation. Even in a home or gym, stashing magnesium carbonate separately just keeps life simple and safe.
Permanent, easy-to-read labels aren't just a good habit—they keep people safe and keep the material where it belongs. I've seen mix-ups from faded or missing labels that meant wasted supplies. Write the date received, and cycle older stock to the front—a trick borrowed from anyone who’s worked in restaurant kitchens. Taking out just what you need and sealing the rest within minutes leaves far less chance for trouble. Small steps, big payoff.
A climate-controlled room goes a long way, but most people don’t store chemicals in a lab-grade vault. A dry indoor closet, away from windows and water pipes, usually beats out a musty garage or utility room every time. Regular checks on containers spot leaks before the mess happens. Keep powders dry, don’t mix storage with reactive stuff, and rotate supplies. These basics won’t just save money; they keep people and equipment safe, whether in a science classroom or a climbing gym.
Magnesium carbonate isn’t just a fixture in climbing gyms or a filler in tablet bottles. Across pharmacies, it pops up in antacid shelves, often mixed with other compounds. People grab it from the shelf because the promise seems simple: combat that burning acid reflux fast. On the surface, the logic seems solid. This mineral reacts chemically with stomach acid, helping to calm that familiar chest burn. Older relatives have sworn by it since the sixties. Today, millions reach for magnesium-based relief hoping for something gentler on the gut than chalky aluminum or sodium-heavy options.
Doctors and pharmacists have watched people try everything for heartburn. Those with chronic reflux or sensitive stomachs sometimes land on magnesium carbonate. Several antacids rely on it to neutralize hydrochloric acid short-term. People trust it for the reason kids trust their grandparents’ remedies—magnesium is a natural part of many bodies and diets. Clinical studies confirm the neutralizing action, at least for mild symptoms. A 2022 review in the World Journal of Gastroenterology highlights the compound’s effectiveness against acid without the risks of severe constipation seen with calcium or kidney strain associated with aluminum-based products.
Folks with heart or kidney issues need to pay attention. Even over-the-counter doses can push up blood magnesium, especially in people with chronic kidney disease. Toxicity from a few tablets is rare, but more risk comes from chronic overuse or high-dose supplements. Constipation and diarrhea both can pop up—magnesium often tips digestion toward the loose side, a well-known side effect.
One issue that’s hard to ignore: self-medicating frequently masks bigger problems. A few tablets after pizza usually won’t hurt, but daily use deserves a professional checkup. Gastrointestinal symptoms can signal everything from food intolerances to ulcers or infections. Popularity doesn’t always mean safety—magnesium-based antacids gained so much traction partly because they’re cheap and have been around for decades.
Antacids make sense when you’re dealing with the occasional greasy meal fallout. Real prevention happens outside the medicine cabinet. Avoiding late-night snacks, cutting down on spicy foods, keeping your gut healthy with a balanced diet, and managing stress tackle heartburn where it starts.
Doctors recommend clear limits: short-term use, check labels for magnesium content, and never swap a chronic prescription for daily OTC antacids. Dietary sources—green leafy vegetables, nuts, seeds—give plenty of magnesium safely, and there’s no substitute for figuring out which foods trigger your symptoms.
Magnesium carbonate’s place on the pharmacy shelf is earned, but it’s not a miracle. Reviews paint a picture of reliable, safe relief in moderation, with the caveat that sensitive populations—especially seniors and those with kidney trouble—should check in with their providers. Antacids will always have a place for mild episodes, but nobody benefits from masking a more serious or persistent problem. Like any tool, magnesium carbonate works best as part of a bigger plan, not a daily crutch.
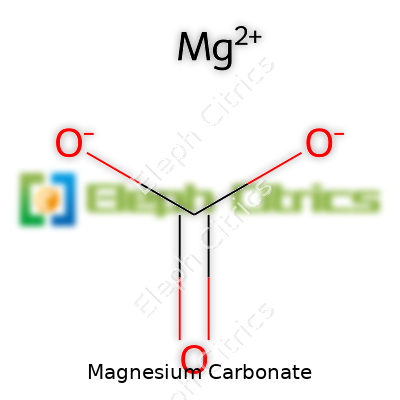

| Names | |
| Preferred IUPAC name | Magnesium carbonate |
| Other names |
Carbonic acid magnesium salt Magnesite Barringtonite Nesquehonite Hydromagnesite Chalk (magnesium) MgCO3 |
| Pronunciation | /mæɡˈniːziəm ˈkɑːbəneɪt/ |
| Identifiers | |
| CAS Number | 546-93-0 |
| Beilstein Reference | 035142 |
| ChEBI | CHEBI:32599 |
| ChEMBL | CHEMBL1201092 |
| ChemSpider | 23821 |
| DrugBank | DB01377 |
| ECHA InfoCard | ECHA InfoCard: 100.029.173 |
| EC Number | 208-915-9 |
| Gmelin Reference | Gmelin Reference: 1732 |
| KEGG | C07704 |
| MeSH | D008271 |
| PubChem CID | 109333 |
| RTECS number | OM3850000 |
| UNII | 7UJQ5OPO28 |
| UN number | UN1414 |
| Properties | |
| Chemical formula | MgCO₃ |
| Molar mass | 84.313 g/mol |
| Appearance | White, odorless, and tasteless powder |
| Odor | Odorless |
| Density | 2.958 g/cm³ |
| Solubility in water | Insoluble |
| log P | -0.5 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 4.5 |
| Basicity (pKb) | 8.52 |
| Magnetic susceptibility (χ) | −18.9·10⁻⁶ |
| Refractive index (nD) | 1.509 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 65.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1095.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1095.8 kJ/mol |
| Pharmacology | |
| ATC code | A02AA01 |
| Hazards | |
| GHS labelling | GHS07 Warning |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture. |
| Precautionary statements | P264, P280, P302+P352, P305+P351+P338, P332+P313, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0-W |
| Autoignition temperature | 350°C |
| Lethal dose or concentration | LD50 oral rat 8,400 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral-rat LD50: 8,500 mg/kg |
| NIOSH | MN0800000 |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 15 – 30 mg/kg |
| Related compounds | |
| Related compounds |
Magnesium oxide Magnesium hydroxide Magnesium bicarbonate Calcium carbonate Sodium carbonate |