Malonic acid found its chemical identity in the 19th century when Justus von Liebig and Auguste Cahours first prepared the compound. Early chemists didn’t just come across malonic acid by accident—they chased after new acids by oxidizing various organic substances, often not sure what they would discover. Once it became clear this simple dicarboxylic acid had real potential in synthesis, researchers moved beyond just cataloging its existence. The stage for synthetic organic chemistry changed dramatically; suddenly, people had a building block that opened up fresher avenues for drug and dye creation. Experimental procedures from those early days, like the malonic ester synthesis, made it into chemistry textbooks and still get taught to students experimenting with carbon-carbon bond formations.
Malonic acid stands out for one reason: it works both as a reagent for reactions and as a component in manufacturing. Its structure, an unassuming three-carbon backbone with two carboxylic acid groups, lets it act as a starting material for pharmaceuticals, vitamins, fragrances, and specialty polymers. It shows up as a white crystalline powder, dissolves smoothly in water, and stores well if kept dry and away from strong bases or oxidizers. Chemical suppliers package it in high-density polyethylene containers or bags depending on the order size, always sealed to keep water out. People working in different fields—chemists, materials engineers, and researchers—value it because it sits at the crossroads of several supply chains and science labs.
Malonic acid wears its basic physical traits on its sleeve: it melts at about 135°C, boils above 140°C where it actually decomposes, and weighs in at 104.06 grams per mole. The compound dissolves well in water and polar solvents thanks to those carboxy groups. Because of the closely situated acid groups, it gives up its protons a bit easier than acetic acid or succinic acid, with pKa values around 2.83 and 5.69. What really sets malonic acid apart is its potential for forming esters and other derivatives. Drop some heat and a dehydrating agent into the mix, and malonic acid smoothly breaks down to carbon dioxide and acetic acid. It doesn’t put on airs—its reactions come down to classic acid chemistry, like decarboxylation and condensation, that form the backbone of many synthetic methods.
Manufacturers keep a close eye on technical specs, particularly purity and moisture content. Most commercially available malonic acid grades sport purity above 99%, verified with titration and chromatography methods. Moisture must stay below the 0.5% mark to stop clumping, with certificates of analysis listing exact measurements. Packaging, as required by hazmat regulations, includes clear hazard pictograms and the UN number 3261—a heads-up to anyone handling or shipping it. Proper labeling means lot number, expiration date, and safety warnings are all there in plain view. This careful tracking ensures traceability from synthesis all the way to application, and all documentation follows the latest GHS labeling standards.
Labs and plants typically produce malonic acid by hydrolyzing diethyl malonate with strong acid or base. The process starts with heating diethyl malonate—an ester itself formed from the reaction of ethanol and malonic acid or malonyl chloride—in a mix of water and acid or sodium hydroxide. Hydrolysis cleaves the ester bonds, and after acidifying the solution, pure malonic acid crystallizes out as temperatures drop. This tried-and-true process keeps things scalable, converting relatively cheap starting materials into a highly sought-after compound. For large-scale manufacturers, process control systems keep the acid and base doses precise, collect data on yield, and implement in-line filtration to scoop out impurities before drying and grading the finished acid crystals.
Anyone who has taken a college-level organic chemistry course has probably met the malonic ester synthesis. Malonic acid or its esters join with alkyl halides under basic conditions—usually sodium ethoxide in ethanol—then undergo decarboxylation with a bit of heat. This method grants chemists a straightforward way to tack on two carbon atoms in a single step. Malonic acid also takes part in Knoevenagel condensations, coupling smoothly with aldehydes to create a family of alpha,beta-unsaturated compounds crucial in both fragrance and pharmaceutical industries. On top of that, it functions as a precursor to barbiturates and vitamin B1 synthesis. In the world of polymer chemistry, cross-linking reactions allow malonic acid to give rise to biodegradable polymers, which builds new momentum for sustainable materials and green chemistry.
Chemists and suppliers don’t speak in code, but malonic acid moves under an unusual crowd of names. Among them: propanedioic acid, dicarboxymethane, and just ‘malonate’ when in its ionized form. On bottles or bags, one might see the EINECS number 204-673-3, or the EC label 607-095-00-3, granting another layer of traceability in global markets. Sometimes, product literature uses “ethane-1,3-dioic acid,” especially in technical documentation originating in Europe. Each synonym gets tied back to the core structure that’s familiar across reference databases and regulatory agencies, so anyone in the loop avoids confusion whether they’re discussing regulations, applications or procurement.
Working with malonic acid doesn’t demand elaborate hazmat suits, but basic PPE rules apply. Gloves, lab coats, and goggles keep skin and eyes away from this mild but corrosive solid. Inhalation of dust or splashes to eyes irritate, so fume hoods and eye wash stations make labs that much safer. Transporters follow the UN Dangerous Goods Code, classifying malonic acid under Class 8 corrosives. Material Safety Data Sheets—audited according to latest GHS standards—warn about storing it far from bases, oxidizers, and anything that gives off heat. In the workplace, standard operating procedures focus on proper weighing, storage in cool, dry spaces, and immediate cleanup of spills to avoid cross-contamination with other chemicals. Personnel receive annual safety refreshers; near misses or exposure get logged for future prevention.
Malonic acid acts as more than just a chemistry curiosity. In pharmaceutical synthesis, it forms the spine for drugs like barbiturates, antihypertensives, and B-complex vitamins. Its ability to extend carbon chains, especially via ester intermediates, helps drug developers tweak molecular activity for better therapeutic speed and safety. Agrochemicals, including plant growth regulators and certain herbicides, rely on malonic acid as an intermediate, too. Outside of healthcare, it stakes a claim in flavor and fragrance creation, where it tones down harsh aftertastes or rounds out fruit scents. Materials engineers pay attention to malonic acid when developing biodegradable polymer blends, targeting greener packaging solutions or single-use items that break down easily. Recently, research teams started exploring its role as a buffering agent and in nanoparticle surface modification—a shift showing just how adaptable this simple acid stays after more than a century in the limelight.
Research on malonic acid doesn’t sit on the shelf. Scientists view it as an old standby for chain-elongation reactions but keep finding fresh pathways to turn it into complex molecules. People have published studies using malonic acid to synthesize advanced photosensitive compounds or to chelate toxic metals from wastewater. Pharma researchers work with it to speed up the manufacture of anti-cancer agents, reducing steps and minimizing solvent waste. Nanotechnology specialists use its carboxylic groups as anchors for silver and gold nanoparticles, boosting the stability of medical imaging inks or electronic components. Beyond synthesis, universities test new catalysts to improve the yield of malonic acid from cheaper biomass sources, broadening supply beyond petrochemical feedstock.
On the toxicity front, malonic acid demands respect more than outright fear. Ingested or inhaled, it can irritate mucous membranes, eyes, and skin. Rodent studies show higher doses disrupt the Krebs cycle, blocking energy production at the cellular level. Chronic exposure links to mild metabolic issues, but short, low-level contact typically causes nothing worse than discomfort or localized skin redness. Most toxicity studies focus on acute exposure, with a consensus that regulatory thresholds for workplace air stay below 1 mg/m³. The acid's irritant effect keeps workplace safety regulations front and center, especially as new applications in biomaterials or consumer goods come into play. Disposal remains a priority too, as waste malonic acid shouldn’t enter waterways—neutralization with base and centralized industrial treatment provide the safest route.
Looking ahead, malonic acid’s potential rides on two main trends: green chemistry and advanced pharma manufacturing. Biotechnologists see new value in fermentative or enzymatic routes to malonic acid—avoiding the energy-hungry petrochemical processes of the past. As the pressure increases for more sustainable sources, plant-based and microorganism-driven production could reshape how the industry supplies malonic acid globally. On the industrial chemistry front, the push for fewer reaction steps and better atom economy aligns well with malonic acid’s proven record in carbon chain construction. Demand from the biodegradable plastics sector points toward significant volume increases as bans on single-use plastics pick up. Pharmaceutical innovators, chasing new scaffolds for anti-infectives and targeted therapies, find malonic acid a consistent performer due to its reactivity and simplicity. Research grants and venture capital have begun funneling into startups and university groups targeting better, cleaner malonic acid routes—setting the stage for new breakthroughs that couple old-school chemistry reliability with 21st-century needs.
People outside laboratories rarely hear much about malonic acid, but chemists appreciate its range of talents. Malonic acid, a dicarboxylic acid by structure, delivers more than a complicated chemical name. In a real sense, it’s a tool that opens doors to building more complex compounds. Anyone who’s tried even a basic chemistry set will remember that building blocks make the game. Malonic acid gives researchers and industrial scientists a reliable way to build carbon bonds, a fundamental move for synthesizing drugs, flavors, dyes, and more.
Take pharmaceuticals as an example. Many common medications depend on malonic acid or its derivatives during manufacturing. Barbiturates—old but still important sedative drugs—view malonic acid as the starting point. The barbiturate backbone, used in seizure medications and anesthesia, wouldn’t exist without it. Knowing that a single compound sits behind therapies that still save lives shifts the way we value these humble acids.
Outside the world of clinical drugs, the acid crops up in agrochemical research too. Pesticides and plant growth regulators need reliable synthesis, and malonic acid helps by offering stable, predictable reactions. Growing up on a small farm, I never imagined the chemistry behind crops, but now I realize how raw ingredients like this can influence what actually reaches our plates.
Malonic acid pops up where least expected. In the food industry, it becomes a flavor intermediate, helping labs develop new synthetic tastes for candies and packaged snacks. It's not about adding the acid directly to food, but shaping the flavor molecules many take for granted. Even in the polymer industry, malonic acid plays a subtle but real role. Many plastics, adhesives, and resins gain their properties from building blocks first made using malonic acid. If you’ve ever used strong glue or a tough plastic container, odds are good some molecule along the way met malonic acid in the process.
One major draw of malonic acid lies in its gentle environmental footprint compared to some heavier-weight synthesis chemicals. Standard malonic acid degrades with less fuss—breaking down into nontoxic pieces without stubborn residues. That’s crucial for the future of green chemistry. Anyone invested in lowering chemical waste can appreciate a molecule that doesn’t clog up waterways or stick around in dangerous forms.
Still, nothing scores a perfect environmental record. Large-scale production of malonic acid from petroleum raises concerns. Sustainable alternatives, such as bio-based production using fermentation or biocatalysts, could make a big difference. The industry faces real incentives to move in that direction, not only because clients and governments demand it, but because costs can shift with cleaner sources. Experience working with small biotech start-ups shows me how switching to greener feedstocks is not just wishful thinking anymore.
Teaching students about malonic acid introduces the concept that even plain molecules carry wide influence. Encouraging more organic chemistry coursework in schools could help spark curiosity about these overlooked chemicals. For folks in manufacturing, pressure continues to build for greener synthesis, so adopting enzyme-driven methods or plant-based feedstocks presents a reasonable path. Governments and industry groups can expand research grants for bio-based methods, steering both science and industry toward solutions that actually involve fewer trade-offs down the line.
Many labs keep malonic acid stocked for good reason. It offers value in synthesis, analysis, and everyday chemistry classes. As someone who has handled it on the bench in university and industry, I’ve always been aware of its dual nature. It looks like a harmless white powder, but some care is required because appearances don’t always reflect the real story.
Malonic acid’s dangers come from its chemistry. It can irritate skin and eyes, and dust makes inhalation something to avoid. I’ve watched classmates cough from just a light breeze carrying dust across a fume hood. NIOSH and OSHA haven’t set firm legal limits for it, but that isn’t a free pass. Material safety data sheets cite irritation, nausea, and even breathing problems as risks. Gloves, goggles, and a decent lab coat reduce the risk, but I always take dust seriously.
If you get it on your hands, a quick rinse with lots of water stops most irritation. I accidentally rubbed my nose after handling some without gloves once and felt a strong sting that lasted half an hour. That taught me respect for all lab chemicals, not just the glamourous poisons and acids people hear about in movies.
Malonic acid is not considered a carcinogen, and there's nothing suggesting it causes chronic problems at normal lab exposures. No solid evidence points to unique hazards beyond usual acid risks. Still, people with asthma or sensitive airways may want to speak up before working closely with it. Dust and acid vapor might make it harder to breathe for some folks, especially in a poorly ventilated space.
Safety comes down to consistent habits. Running a clean workspace, disposing of waste properly, keeping containers sealed, and careful weighing matter more than people think. I’ve seen minor spills escalate because someone tried to sweep up powder too quickly, sending fine particles flying. Damping it down with wet towels before sweeping keeps the dust out of the air.
Sharing tips among coworkers adds another layer of safety. Someone always finds tricks to keep a workspace tidy and hazard-free. One tech in my lab brought in a small vacuum equipped with a HEPA filter—the simple change kept our benchtops and even the floor safer. Open communication and a bit of peer oversight remained our best tools to avoid accidents.
To lower risks, switching to chemicals with even less toxicity helps if the application allows it. Some reactions can't drop malonic acid, but newer protocols sometimes provide the same results with less harsh compounds. Training newcomers in both the risks and cleanup procedures also helps. I remember a professor insisting everyone run a full safety drill before starting the semester. It felt tedious, but paying attention stopped disasters later.
In short, treating malonic acid with basic respect and not cutting corners makes the difference. A culture of safety, some down-to-earth reminders, and steady hands keep labs running smooth—even when the chemicals aren’t entirely innocent.
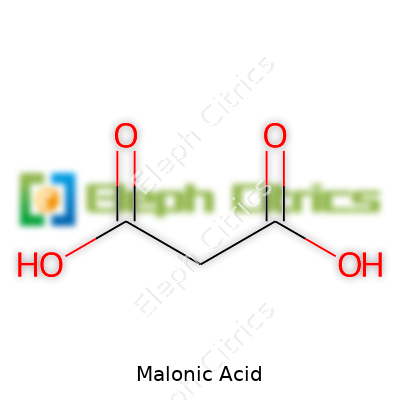
Malonic acid carries the chemical formula C3H4O4. In everyday terms, that means three carbon atoms, four hydrogens, and four oxygens come together in one molecule. Most of us probably never gave this combo much thought outside of a classroom. But think about this: those atoms form part of the building blocks that let organic chemistry branch off in dozens of directions.
The first time I heard about malonic acid was during an introductory chemistry class. Our professor pointed out that it’s not just another acid on the shelf; its structure opens up doors for making more complex molecules. C3H4O4 fits a chain of carboxylic acids, meaning it can help scientists build up or break down larger organic items. That gets used in many drug syntheses and even in the production of vitamins. The everyday relevance impressed me — it didn’t need a fancy pedigree, just a practical spot in underlying chemical reactions that touch everything from food science to crop protection.
What stands out about malonic acid lies in its “dicarboxylic” nature. Two carboxyl groups, one at each end, let this acid act a bit like a chemical connector. These groups make it quite reactive, eager to pair up or swap atoms in several reactions. Laboratory protocols involve malonic acid for Knoevenagel condensations where its structure enables careful control over products, especially for custom synthesis or scale-up projects.
In pharmaceutical settings, chemists turn to its chemical formula because it supports building diverse drugs. Used the right way, C3H4O4 can offer up carbon skeletons for everything from pain relievers to stabilizers. In plant science, malonic acid’s formula helps in investigating natural metabolic cycles, contributing to our broader understanding of how plants respond to different environments.
Even though malonic acid looks simple, handling it needs care. Too much exposure can irritate skin or eyes, so following lab safety is not just official talk — it keeps everyone healthy. Safe procedures matter, and it’s worth keeping up to date on storage and disposal standards. The world doesn’t need more accidental spills or chemical mishaps in university labs or factories.
Production requires best practices too. Factories making malonic acid often apply greener chemistry approaches, lowering toxic byproducts or recycling leftover reactants like cyanoacetic acid. This cuts down on environmental impact. Regulatory measures help push for these steps, and they’re nothing to shrug off.
It’s easy to overlook a three-carbon acid like this, but C3H4O4 underpins work in pharmaceuticals, agriculture, and chemical research. Growing interest in sustainable processes encourages the industry to adopt bio-based routes and energy-efficient synthesis. Investment in research and industry standards means safer labs, cleaner products, and a future where foundational compounds can keep building on what’s already reliable.
Malonic acid shows up in labs and chemical supply rooms everywhere. If you’ve ever handled this stuff, you probably know it’s not just another bottle to toss on any old shelf. It can decompose and give off carbon monoxide. Forgetting about it or ignoring storage advice could invite serious safety issues or spoil your project materials. Every chemistry student hears the horror stories—one leaky container, and you’re scrambling to clear the room.
Walk into a well-managed lab, and cold, dry storage is the rule for organic acids. Malonic acid fits right into this routine. The worst mistakes happen when folks treat it like table salt, leaving it out in the open. Exposing it to humidity or heat usually guarantees clumpy, degraded powder and a faintly acidic smell that never seems to come out of the air.
Skip these headaches by keeping the bottle tightly sealed, away from direct sources of moisture or intense sunlight. Glass or high-grade polyethylene bottles with screw caps hold up well and keep the acid stable. Store it below room temperature—most labs rely on refrigerators between 2 to 8 degrees Celsius. This low temperature keeps decomposition at bay. I’ve seen acid go bad when left on a warm shelf, and it never ends well for the experiment or the clean-up crew.
Once, I watched a group of first-year students pile up a mountain of chemical bottles in a stuffy closet next to a water heater. One cracked at the rim and nobody noticed until days later, when a sharp, sour odor crept under the door. Liquid pooled on the floor, making cleanup a real pain and costing time and money. After that, our supervisor banned malonic acid from warm supply rooms forever.
Labels matter. The fastest way to create confusion is to scribble half-words and toss a bottle back on the shelf. Clear, neat labeling does more than keep things tidy—it saves you from emergency calls because someone grabbed the wrong chemical. Spell out the date opened and the shelf life. It sounds tedious, but the next person in line will thank you.
Proper training cuts down on accidents. A solid introduction to safe chemical storage gives people the backbone to speak up before something goes wrong. After years in teaching labs, I’ve learned that reminders at the start of every semester set the tone. People won’t stick to safe habits if they don’t understand the consequences.
Fire extinguishers, fume hoods, and up-to-date materials safety data sheets serve as your backup. Aside from cold, dry storage, an easy-to-reach spill kit helps. You hope never to use it, but it’s reassuring once you see white powder on a countertop.
Ignoring safe storage takes away the predictability everyone counts on in science. Malonic acid won’t break the budget, but losing a batch wastes resources and risks health. A few extra minutes checking temperature, humidity, and labels mean fewer accidents and more reliable research. The habits built around simple storage routines save money, protect people, and keep research moving forward. That’s a trade-off worth making every day.
Malonic acid pops up in plenty of chemistry labs. Its uses range from synthesizing barbiturates to tweaking pH in research settings. While it might sound like something reserved for industrial beakers and scientific journals, its reach extends to many classrooms and specialty shops—though it’s not something you’ll find at your average grocery store.
My own experience with organic chemicals started in a university lab. Before ordering even simple acids online, paperwork and oversight shaped every step. Malonic acid carries no mysteries about its chemical makeup, but stores and wholesalers must follow strict protocols. The reason? It can be used to produce compounds of concern and that triggers both safety and regulatory barriers.
Many folks try online marketplaces—Amazon, eBay, or specialty chemistry suppliers. Sellers may list it, but actual purchase hinges on checking your credentials. More and more, these platforms require you to prove educational or business ties to science. I once tried to help a friend buy a reagent from a U.S. supplier for a personal project. Their support asked for a university email, a purchase order, and a signed user agreement to ensure it wouldn’t end up misused. Access remains limited for non-professionals to help steer clear of legal problems.
Academic researchers or those with a legitimate business angle can turn to large chemical suppliers. Sigma-Aldrich, Thermo Fisher, and Alfa Aesar dominate the space. Their process includes background checks and shipment tracking. Sizeable distributors won’t touch unregistered buyers. For high school teachers, colleges or those partnered with research institutions, this process gets easier with institutional accounts.
Malonic acid is less hazardous than many other chemicals, but it still comes with health risks. Swallowing, inhaling, or even touching can bring on discomfort. Labs need practical controls: fume hoods, gloves, even face shields. Many home buyers overlook this. Risk rises when folks try working outside supervised lab spaces.
Community forums sometimes buzz with “workarounds” for getting lab chemicals. Shortcutting safety, or lying about a planned use, stands to cause more harm than good. Inexperienced hands can lead to spills, mixing errors, and, rarely, even legal trouble.
Science education thrives on hands-on access, but safety can’t get tossed aside. One practical step starts with schools and amateur chemistry clubs banding together for legitimate, bulk purchases. Pooling demand can clear bureaucratic hurdles and ensure everyone gets supervised handling. Building relationships with local universities can also open access for educational experiments in controlled environments.
For folks outside institutions, raising public awareness about purchasing challenges can push policymakers toward better systems. Streamlining the application process without slackening controls would expand curiosity-driven research and keep dangerous substances out of the wrong hands. Open, ongoing dialogue between suppliers, regulators, teachers, and amateur scientists makes progress possible. Even if I can’t buy malonic acid today as a non-affiliated hobbyist, efforts to bridge this gap matter for tomorrow’s problem-solvers.
| Names | |
| Preferred IUPAC name | propanedioic acid |
| Other names |
Propanedioic acid Methylenemalonic acid Dicarboxymethane |
| Pronunciation | / məˈlɒnɪk ˈæsɪd / |
| Identifiers | |
| CAS Number | 141-82-2 |
| Beilstein Reference | 1208737 |
| ChEBI | CHEBI:30794 |
| ChEMBL | CHEMBL1402 |
| ChemSpider | 565 |
| DrugBank | DB04248 |
| ECHA InfoCard | 03b2c2af-dde0-4986-835a-11361fa23c59 |
| EC Number | EC 200-007-1 |
| Gmelin Reference | 8596 |
| KEGG | C00183 |
| MeSH | D008318 |
| PubChem CID | 867 |
| RTECS number | OO5250000 |
| UNII | 7U8FA37353 |
| UN number | UN1329 |
| Properties | |
| Chemical formula | C3H4O4 |
| Molar mass | 104.06 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.619 g/cm³ |
| Solubility in water | 763 g/L (20 °C) |
| log P | -0.81 |
| Vapor pressure | 0.00012 mmHg (25°C) |
| Acidity (pKa) | 2.83, 5.69 |
| Basicity (pKb) | 1.48 |
| Magnetic susceptibility (χ) | -44.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.430 |
| Viscosity | 2.7 mPa·s (25 °C) |
| Dipole moment | 5.62 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 157.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -889.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1341.9 kJ/mol |
| Pharmacology | |
| ATC code | A16AA15 |
| Hazards | |
| Main hazards | Harmful if swallowed, causes serious eye irritation. |
| GHS labelling | GHS07, GHS05 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H302, H315, H319, H335 |
| Precautionary statements | P264, P280, P301+P312, P330, P305+P351+P338, P337+P313, P501 |
| Flash point | 140 °C |
| Autoignition temperature | 222 °C (closed cup) |
| Explosive limits | Not explosive |
| Lethal dose or concentration | LD50 oral rat 1600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral (rat) 1600 mg/kg |
| NIOSH | GF5950000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) of Malonic Acid: "TWA 1 mg/m³ |
| REL (Recommended) | 5 kg |
| Related compounds | |
| Related compounds |
Acetic acid Succinic acid Dibromomalonic acid Diethyl malonate Formic acid |