Digging through chemical history, one will see manganese compounds following the arc of industrial and agricultural advances. Farmers and chemists have always needed manganese in trace amounts for plant nutrition and enzyme activation. For this, manganese lactate made its way from lab oddity to a staple in nutritional science. Chemists spotted the convenience of pairing manganese with lactic acid—nature’s own fermentation product—then started isolating it for supplements and lab use. By the late 20th century, demand extended to food fortification, animal feeds, and research settings. Prominent chemistry handbooks flagged manganese lactate as more soluble and bioavailable compared to some raw manganese salts. Public health recognized its value in diets missing trace minerals.
Manganese lactate typically appears as a white to off-white crystalline powder. Most manufacturers target pharmaceutical or analytical grades, which require a purity north of 98%. This compound’s main job has come down to supplementing diets or laboratory science when calcium blockers and alternatives fall short. Pharmaceutical firms and research labs prize it for predictable absorption in both humans and animals. In the food sector, it appears as a trace mineral fortifier in custom blends for processed foods.
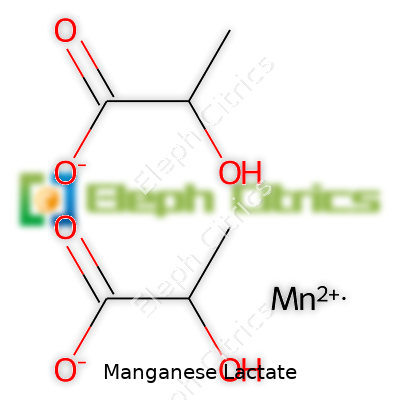
This compound’s full name is manganese(II) lactate, with the formula Mn(C3H5O3)2. CAS number 15827-60-8 marks it out on chemical registries. It dissolves easily in water, starts to decompose if heated past 200°C, and does not play well with strong oxidizers. Thanks to manganese sitting at the center, the solution often picks up a faint pinkish hue—an easy way to tell it apart from colorless lactates. Commercial samples stay stable under dry, cool storage. This low toxicity means shipping and packaging follow lighter regulations than more caustic metallic salts.
Each batch arrives with a certificate outlining manganese content (commonly around 16-18%), moisture content, and presence of contaminants like heavy metals. Leading brands put special effort into batch-to-batch consistency and traceability. Packing materials must keep moisture out, as the compound shows hygroscopic tendencies if left in a humid warehouse. On containers, regulations demand clear labels: trade name, net weight, lot number, and expiry date. Some regions ask for hazard pictograms and disposal advice, reflecting moves toward greener manufacturing.
Manufacturers usually react manganese carbonate or manganese oxide with lactic acid under controlled pH and temperature, continuously stirring until the mixture stabilizes. They avoid chlorides and sulfates to sidestep unwanted byproducts. Once the reaction finishes, workers filter, evaporate, and dry the product—taking precautions against straw-yellow impurities that hint at overheated material. Chemists must balance speed and purity, since quick-dried batches can show dark spots from caramelized organic acids, while slow methods may bump up process costs.
At its simplest, manganese lactate works as a mild reducing agent, and under the right pH, it can swap out manganese for another bivalent metal in double decomposition reactions. In soil science, chemists study how the lactate backbone assists manganese absorption via chelation effects. When exposed to strong acids, the lactate decomposes, releasing free lactic acid and divalent manganese ions. Industrial researchers sometimes experiment with amino-acid modifications to tweak absorption and reactivity for animal health, blending it into larger chelate complexes.
In technical literature, manganese(II) lactate appears under various guises, including Manganous lactate, Manganese lactic acid salt, and Lactic acid manganese(II) salt. Some supplement brands slap on proprietary names or blends, sometimes pairing manganese lactate with other micronutrients. Trade catalogs often group it with similar compounds like calcium lactate and zinc lactate due to overlapping industrial applications.
Used in typical concentrations, manganese lactate shows only mild risks. Inhalation of dust or accidental ingestion by children poses minor irritation hazards—prompting standard safety data sheets to advise gloves and dust masks during handling. Teams keep workspaces clean to avoid cross-contamination in food or pharmaceutical blending environments. Disposal guidelines send unwanted product to general chemical waste, as long as it doesn't pick up contaminants on the shop floor. Regulatory bodies like the FDA and EFSA monitor dietary manganese closely, setting strict limits for both supplements and food additives to sidestep chronic overexposure.
Feed mills and premix plants rely heavily on manganese lactate to round out animal diets—helping livestock avoid skeletal defects and metabolic slowdowns often tied to low manganese absorption. Processed food manufacturers blend it into grain and dairy products lacking trace minerals post-refinement. Biomedical research groups use it to explore metabolic pathways and oxidative stress markers linked to manganese’s role as a cofactor for enzymes. Agriculture draws from its soluble, plant-available form to supplement hydroponic and traditional crops.
Current research focuses on improving the bioavailability and environmental footprint of manganese supplements. Scientists test novel chelation agents and sources—like fermentation-derived lactic acid—to strike better balance between cost and nutritional benefit. Some labs investigate manganese lactate as a stabilizer for pharmaceuticals or in enzyme-enhancing blends for clinical nutrition. Public health researchers pay attention to population studies linking mineral deficiencies to chronic diseases, spotlighting products that can fill nutritional gaps without causing overloading.
Toxicologists have checked manganese lactate’s behavior in both acute and chronic exposure scenarios. At approved doses, the body manages manganese excretion efficiently, especially when ingested with food. Poisoning or cumulative neurotoxicity arises at levels far above what food fortification supplies. Experts track manganese’s role in neurodegenerative processes, notably in occupational settings, watching for early warning signs such as motor function changes or shifts in blood chemistry. No current body of evidence singles out manganese lactate as riskier than other supplements, but overuse remains a background concern for regulators.
Looking ahead, production methods will continue getting greener and safer, thanks to stricter waste limits and pressure for sustainable sourcing. Markets will push mineral blends further, especially those supporting personalized nutrition and precision agriculture. Researchers keep probing the interface between plant science, animal nutrition, and human health, hunting for new combinations and applications for manganese compounds with better absorption and lower environmental impact. As a long-term staple in both bulk and specialty chemical markets, manganese lactate’s story carries on as nutrition and technology keep evolving side by side.
Manganese lactate looks like just another chemical name from high school chemistry, but it plays a real part in keeping both living things and businesses running. This compound combines manganese, a trace mineral, with lactic acid–you see the “lactate” in the name. Manganese shows up naturally in nuts, grains, and leafy greens, but sometimes, diets or processes just fall short.
Doctors and nutritionists talk about manganese for good reason. The body needs it every day, just not in huge amounts. It’s part of enzyme systems, and these enzymes run important tasks: bone strengthening, taking care of nerves, and processing carbs and fats. When manganese comes in a lactate form, the body absorbs it more easily than from rocks or metals.
Some folks have conditions that hurt nutrient absorption. For them, pills or drinks with manganese lactate fill in the gaps, especially for those on restricted diets or recovering from illness. Athletes, too, sometimes rely on supplements like this to help keep bones tough and metabolisms running smoothly under stress.
A healthy farm needs more than sunshine and water. Animals, especially those in big operations, depend on balanced feed to stay strong and productive. Feed makers often add manganese lactate so livestock, and sometimes even pets, get this mineral in absorbable form.
Chickens, pigs, and dairy cows need manganese for bone growth, for laying strong eggs, and for keeping reproductive systems working. Without it, even with plenty of other nutrients, animals can fall behind in growth or develop weak bones. I’ve seen how the right mineral balance makes a difference on farms, with healthier animals and fewer problems to solve down the road.
Beyond biology, manganese lactate turns up in manufacturing. The food industry uses it as a mineral additive for fortified foods and drinks. Precise control over mineral content helps companies stick to nutrition labeling laws and provide real health value, not just marketing claims.
Water treatment also draws on manganese compounds to help break down contaminants that don’t get caught by simple filters. This isn’t as well-known as using chlorine or fluoride, but it’s important for smaller-scale systems trying to improve water quality in specific situations or locations where regular infrastructure doesn’t reach.
Like many nutrients, manganese helps in small amounts but causes problems if people or animals get too much of it. Regulators keep strict watch on how much goes into animal feeds, supplements, or even food products. There’s been a push to use organic forms like manganese lactate rather than inorganic salts so that less of the element ends up moving into soil or water due to runoff. This reduces the risk of environmental buildup and related health risks for humans and wildlife.
The move toward better nutrition, animal welfare, and cleaner processes keeps demand for safe, well-absorbed forms of minerals high. Manganese lactate fits that need, serving as a bridge between biology and industry. As nutrition science grows and industries look for greener methods, compounds like this will keep playing a quiet but crucial role.
Manganese hardly ever grabs headlines, but in the world of nutrition, it plays a quiet but important role. Our bodies use small amounts of manganese for things like supporting bones, helping enzymes do their jobs, and fighting off oxidative stress. It shows up naturally in foods like whole grains, nuts, tea, leafy vegetables, and even some fruits. Meeting daily needs isn’t especially tricky for most folks; the average diet supplies what’s needed.
Supplements add a twist. Not everyone pulls enough minerals from food, so some turn to capsules or fortified foods. That’s where manganese lactate appears. The compound combines manganese with lactic acid, aiming to boost absorption. Companies add it to multivitamins or sometimes to foods to address potential shortfalls.
Plenty of research says that manganese, at low levels, is not a concern. The U.S. National Institutes of Health set the recommended dietary allowance for adults at around 2.3 mg for men and 1.8 mg for women each day. Excess comes from supplements, not salads, so multivitamin users need to pay closer attention.
Scientific studies suggest manganese lactate isn’t more dangerous than other forms like gluconate or sulfate, as long as folks stick with proper serving amounts. Medical literature shows the human body handles it well in small doses, using its own controls to get rid of extra manganese through bile and eventually out of the system. The European Food Safety Authority and the U.S. Food and Drug Administration both treat manganese additives as generally recognized as safe when companies follow guidelines.
Problems show up only with large amounts—far above what someone would get through standard supplements. Chronic overconsumption may cause harm, mainly affecting the nervous system and leading to symptoms that look a bit like Parkinson’s disease. This mainly happens in workplaces with heavy airborne manganese dust, not at the breakfast table.
Real peace of mind starts with trusting the product label. If someone’s picking a multivitamin, it matters to check whether extra manganese is listed, and how much. A few years ago, I met people dedicated to those "mega" minerals, taking multiple supplements daily. Many didn’t realize ingredients could overlap, creeping up out of the safe zone. That’s where informed decisions come in.
People with liver trouble need extra caution, because their bodies process manganese less efficiently. Children face higher risk of overload at lower intakes compared to adults because their brains are still growing. Smart supplementation always looks at age, health status, and total dietary sources together—not in isolation.
The safest path brings together personal responsibility and honest product labeling. Regulators could require clearer manganese content statements, especially in products for children or vulnerable groups. Physicians and dietitians can play a bigger role by asking about supplement habits, not just diet. Making sure people know to avoid double-dosing across different pills keeps intakes well below risky territory.
Eating a variety of foods, limiting excess supplementation unless recommended by a healthcare provider, and checking up-to-date guidelines covers most bases. Most folks never need to give manganese lactate a second thought, unless specific advice or health conditions come up.
You’ll find manganese in just about every multivitamin on pharmacy shelves. It’s a trace mineral – you don’t need much, but your body still relies on it. Manganese plays a role in bone formation, blood clotting, and helps keep your nerves steady. It’s also a piece of the puzzle for enzymes that handle metabolism, meaning it helps unlock energy from the food you eat. Not getting enough can cause real issues, but overdoing it carries risks too.
Most health professionals and trusted sources set daily manganese recommendations at 2.3 mg for adult men, and 1.8 mg for adult women. These values come from research carried out across different populations and validated by agencies like the National Institutes of Health and the World Health Organization. It’s important to remember that these amounts refer to elemental manganese, not manganese lactate specifically, so you’ll need to check the supplement label for the actual content.
Manganese lactate is a compound that delivers this mineral in a form the body can use. If you’ve got a bottle at home, look at the supplement facts. The label should spell out how much elemental manganese is in each tablet or scoop. Sometimes doses aren’t clear, so it helps to keep a calculator handy. If a capsule has 10 mg of manganese lactate, it will provide less than 10 mg of elemental manganese, because the rest of the compound is made up of lactate itself.
Most people hit daily manganese targets through food. Whole grains, nuts, leafy green veggies, and legumes all bring decent doses. People who follow heavily processed diets or rely on intravenous nutrition could face deficiencies, though this is rare. Certain health conditions, like absorption disorders, bone diseases, or inherited issues, might push a doctor to recommend supplements.
I’ve seen people reach for supplements under the impression that more is always better. With minerals like manganese, crossing the safe upper limit can sneak up if you don’t pay attention. Long-term high doses — above 11 mg per day for adults, according to the Institute of Medicine — can harm the nervous system and create symptoms that mimic Parkinson’s disease. Workers exposed to airborne manganese dust in industrial settings have taught researchers a lot about toxicity, and lessons from those cases should guide safe supplement use.
Before starting a new supplement, check in with a doctor or dietitian, especially if you’re treating a known deficiency or have children, are pregnant, or live with kidney problems. Manganese builds up slowly. Unlike vitamin C, your kidneys can’t flush out the excess quickly, so a little caution pays off.
If you end up supplementing, stick close to recommended daily amounts unless a healthcare provider sets a higher dose for a medical reason. Track how much you’re getting from your diet and your supplements. If a product label doesn’t show the elemental manganese in each dose, don’t guess. Contact the manufacturer and ask.
Look for products that have been third-party tested or verified, as these checks cut down on contamination and label inaccuracy. Reputable brands share these details openly. Don’t take internet advice from unqualified sources. As with many nutrients, knowledge and moderation go hand-in-hand.
Manganese supports bone health, helps nerves work, and keeps the body handling carbs and fats the way it should. In a regular diet—spinach, pecans, brown rice—most people get enough without thinking about it. Sometimes, though, a supplement like manganese lactate shows up in a daily routine, either for medical reasons or because someone hopes for extra wellness benefits.
Manganese lactate stands as one of several ways to get this mineral. Doctors may suggest it when someone’s body isn't taking in enough from food. But too much of anything brings trouble, and with manganese lactate, that's especially true when supplements get taken without medical guidance.
Short-term, small doses rarely spark problems. Still, side effects can show up if a person’s body starts to store more of the mineral than it needs. In my experience, most people don’t notice issues from occasional, low-dose supplements. Problems start creeping in after long-term use or from high doses.
Digestive upset comes first—abdominal cramps, nausea, sometimes diarrhea. A few people describe a metallic taste that makes swallowing pills tough. High doses can irritate the gut, especially in people with already-sensitive stomachs.
Here’s where real trouble sets in: the brain and nervous system handle manganese poorly in large amounts. Studies show that workers in fields like welding, where they breathe manganese dust, can end up with memory issues, mood changes, and movement problems that look a lot like Parkinson’s disease. It’s rare, but high-dose supplements over months can send levels up and lead to side effects like muscle stiffness or trouble walking.
For most healthy people, kidneys help flush out extra manganese. But if kidneys get weak or someone already has liver problems, the risk rises. The buildup can even affect how kids grow and think, so no one should give these supplements to children without clear reasons.
Allergic reactions very rarely happen—hives, swelling, and trouble breathing have been reported. Anyone with a known allergy to similar compounds should steer clear or talk to their doctor right away.
If someone wants to boost their manganese intake, food always brings fewer risks than pills or powders. For people who still need supplements, sticking with the recommended dose on the label makes a major difference. Always let a healthcare provider know about all supplements taken, especially with pregnancies, chronic illnesses, or other prescriptions, since interactions can sneak up.
Getting levels checked through a blood test doesn’t make sense for most people, but those with ongoing symptoms—tremors, muscle pain, memory troubles—may want to ask their doctor about the possible link. Pharmacists keep up to date on which drugs or supplements clash with manganese; using their knowledge can stop trouble before it starts.
Strict manufacturing rules in many countries cut down on risks of contamination in manganese lactate. Still, choosing supplements from trusted brands makes a difference. Side effects pop up more often with products from questionable sources.
Supplements can't cover for a weak diet or treat disease on their own. Using manganese lactate with care, knowing the side effects, and paying attention to the body's signals keeps health at the center rather than risking problems down the line.
Manganese lactate offers value for everything from supplements to agriculture, and the right storage keeps it doing its job safely. This compound reacts with moisture and oxygen in the air, and that leads to clumping or loss of strength. If air humidity seeps in, you end up with powder that can’t be mixed easily, and its qualities start to change in unpredictable ways.
Early in my own chemistry days, a simple oversight—leaving the lid off a chemical jar for just a morning—ruined the contents before lunch. Manganese lactate wants protection from air and sunlight the same way, because too much heat or direct light works like an accelerator for chemical breakdown. A cool, dry space like a locked storeroom with limited entry works wonders, because temperature swings promote the exact issues you want to avoid: breakdown, contamination, and messes that waste both time and money.
Room temperature conditions under 25°C do best for this material. Letting things get warmer can push reactions forward, and powders clump. I always recommend keeping chemicals away from boiler rooms and windows for that reason. Even storing the material next to a vent can expose it to humidity. Silica gel packets get tossed in with the jars for extra insurance, especially in climates where humidity doesn’t play nice. Very dry materials like manganese lactate can act like a sponge if humidity creeps in, drawing moisture from the air and caking in the process.
Factories often ship manganese lactate in plastic drums or tightly sealed bags for its protection. Any time a bag gets opened, you want to use as much as you can quickly, then seal things back up fast. Once tear is introduced, air sneaks in, so any leftover material belongs in an air-tight container—ideally one that’s labeled with the open date. Trying to save a few minutes by skipping this step usually turns fresh stock into waste.
Good labeling on containers helps keep chemicals organized and safe. Each jar in my old lab had the name, concentration, and open date right up front—no guesswork, no mix-ups. Safety data sheets sat close by for quick lookup. If your crew might worry about exposure, a posted sheet of handling guidelines next to the cabinet takes away doubt and helps people listen to their gut about using gloves or masks if needed.
If a storage area uses open shelving or gets hot from nearby equipment, expect chemical shelf life to shrink. Spilled powders draw insects and sometimes even mold, depending on moisture. Cross-contamination sneaks up if chemicals sit too close together, especially if they have similar color or texture—one quick scoop from the wrong bag, and a batch may need disposal. Storage mishaps also trigger extra costs down the line, as spoiled chemicals can’t do their job and old powder can mess up formulations.
Solid preparation—dry, cool storage, sealed containers, and clear labeling—makes daily handling smoother and safer. Training staff goes a long way, as even the most careful person eventually gets distracted. Prevention always beats workplace cleanup, and in my experience, small up-front investments pay out for months or years. With manganese lactate, understanding how fast things can go off helps you see why these steps deserve daily attention—not just an occasional audit.
| Names | |
| Preferred IUPAC name | Manganese(2+) 2-hydroxypropanoate |
| Other names |
Manganese(II) lactate Manganese dilactate Lactic acid, manganese(2+) salt |
| Pronunciation | /ˈmæŋ.ɡəˌniːz ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 640-72-6 |
| Beilstein Reference | 3585508 |
| ChEBI | CHEBI:86473 |
| ChEMBL | CHEMBL1201653 |
| ChemSpider | 520960 |
| DrugBank | DB14659 |
| ECHA InfoCard | 100.034.594 |
| EC Number | 231-769-5 |
| Gmelin Reference | 145524 |
| KEGG | C01794 |
| MeSH | D008352 |
| PubChem CID | 85922998 |
| RTECS number | OP0450000 |
| UNII | NFY0O13W9F |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C6H10MnO6 |
| Molar mass | 205.07 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 1.74 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.61 |
| Acidity (pKa) | Acidity (pKa): 3.86 |
| Basicity (pKb) | 7.98 |
| Magnetic susceptibility (χ) | +11000e-6 cm³/mol |
| Refractive index (nD) | 1.52 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 230.5 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1789.5 kJ/mol |
| Pharmacology | |
| ATC code | A12CC03 |
| Hazards | |
| Main hazards | May cause respiratory and skin irritation. Harmful if swallowed or inhaled. |
| GHS labelling | GHS07, GHS09 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: Causes serious eye irritation. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 4,180 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 2,000 mg/kg |
| NIOSH | Manganese, inorganic compounds, as Mn, 0.2 mg/m3 TWA (as Mn) |
| PEL (Permissible) | 5 mg/m³ |
| REL (Recommended) | 12 mg (as Mn)/day |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Manganese(II) chloride Manganese(II) sulfate Manganese(III) oxide Lactic acid Calcium lactate |