Chemists identified the value of lactic acid esters centuries ago, but only much later did methyl lactate carve out a space in industrial chemistry. Early organic synthesis work recognized how to produce esters from lactic acid and methanol. By the twentieth century, rising demand for solvents and intermediates drove both laboratory and commercial interest. Reports from the mid-1900s describe methyl lactate as a green solvent option, especially at a time when industry began searching for alternatives to harsher chemicals like toluene or methyl ethyl ketone. Research on renewable chemical synthesis has often started with lactic acid, a cornerstone of fermentation, linking methyl lactate’s roots to both petrochemical and biological sources.
Methyl lactate serves as both a solvent and a chemical intermediate. It commonly presents as a colorless or pale yellow liquid with a mild, sweet odor, which makes it easier to handle in laboratories and manufacturing facilities than many harsher alternatives. The substance falls under the class of esters, bridging alcohols and acids, specifically methyl alcohol and lactic acid. Its widespread use in pharmaceuticals, cleaning products, electronics, and food packaging ties directly to its low toxicity and solvent power for both water-soluble and oil-soluble compounds.
Boiling at about 145°C, methyl lactate stays liquid through a range of processing temperatures, allowing reliable use in industrial plants. It has a molecular formula of C4H8O3 and a molecular weight near 104.1 g/mol. The density at 20°C sits at roughly 1.1 g/cm³, and it dissolves readily in water, alcohols, and most organic solvents. This mix of polar and nonpolar affinity explains why it keeps popping up in cleaning agents, coatings, and inks. Its flash point, just over 53°C, means handling calls for care but doesn’t require elaborate precautions typical for more hazardous solvents.
Manufacturers usually provide methyl lactate in concentrations from 85% to 98%, depending on downstream use. Technical data sheets specify limits for water content, acid number, color, and residue after evaporation. Labels must carry hazard pictograms based on global harmonized systems, often warning about eye and skin irritation—nothing extreme by chemical standards, but enough to keep safety glasses and gloves as standard gear. Packaging includes tight-sealed drums or cans, since the material can draw in moisture and sometimes hydrolyze back to lactic acid and methanol over time. Product batches get a lot number, and certificates of analysis accompany shipments for traceability and quality assurance.
Industry usually produces methyl lactate by direct esterification, where lactic acid reacts with methanol in the presence of a strong acid catalyst, such as sulfuric acid. The process requires careful temperature control, monitoring of water removal to shift the equilibrium, and purification steps that may include distillation. Some biorefineries now produce methyl lactate by fermenting carbohydrates to lactic acid and then transforming it into the ester, a route favored by manufacturers looking to support bio-based chemical supply chains. Catalyst recovery, water removal, and waste reduction represent ongoing challenges, spurring process innovations.
Chemists appreciate methyl lactate’s versatility as a starting point for other valued products. Hydrolysis splits it back into lactic acid and methanol under acidic or basic conditions. Transesterification brings other alcohols into the mix, creating ethyl or butyl lactate, extending the reach of lactic esters. Under hydrogenation, methyl lactate can produce propylene glycol, a major ingredient in plastics and antifreeze. The molecule also features a reactive hydroxyl group, making it a candidate for further derivatization—think of specialty solvents, plasticizers, or biodegradable surfactants.
Names like methyl 2-hydroxypropanoate, lactic acid methyl ester, and lactic acid, methyl ester give a sense of the chemical family. In laboratory and commercial catalogs, searching any of these variations brings up the same compound. Familiar trade names sometimes simplify procurement, but regulatory filings always tie back to the International Union of Pure and Applied Chemistry (IUPAC) name, methyl 2-hydroxypropanoate.
Handling methyl lactate relies on up-to-date Safety Data Sheets and proper PPE. Dermal contact can irritate the skin, and inhalation of vapors—especially in poorly ventilated spaces—can bother sensitive airways. Facilities set up workspaces with fume hoods and emergency showers, not only for methyl lactate but for the acids, alcohols, and catalysts involved in processing. Storage containers need to remain tightly closed and protected from direct sunlight. Globally recognized standards, such as those from OSHA, REACH, or the EPA, cover exposure limits and waste disposal, helping to keep both workers and local environments safe.
Paint and coatings makers look to methyl lactate for its ability to dissolve polymers without introducing hazardous air pollutants. In electronics manufacturing, the chemical acts as a cleaner, stripping flux residues without attacking sensitive substrates or generating hazardous byproducts. Pharmaceutical formulation benefits from its solubility and mild action—excipients and active ingredients dissolve evenly, creating stable solutions and suspensions. The compound features in some food-contact applications, though this requires grapevine-like adherence to purity and migration regulations. These varied uses connect back to methyl lactate’s flexibility and limited toxicity.
Green chemistry drives much of the current research. Academic and corporate labs frequently test methyl lactate as a replacement for solvents with worse profiles, like NMP or acetone. Recent studies have mapped catalytic improvements in esterification, targeting higher yields and energy savings. Companies keep an eye on cost, feedstock sources, and downstream value chains. In my own lab work, switching to methyl lactate often simplified hazardous waste management, and we noticed a drop in reported skin irritation incidents. The trend has moved beyond small-scale trials: full-scale plants look at methyl lactate for closed-loop solvent cycles, further cutting resource use.
Toxicological tests report low acute oral and dermal toxicity. Irritation tends to show up on repeated contact or at higher concentrations, which echoes my impression in weeks spent prepping alcohol esters. Regulators and research groups continue to track metabolites and breakdown products, especially since methyl lactate hydrolyzes quickly in the body and the environment, forming lactic acid and methanol. Inhaled methanol remains a concern with massive exposures, but typical industrial and lab handling procedures keep concentrations well within global occupational safety limits. Chronic exposure studies reinforce the call for protective equipment and proper training but reassure on long-term effects at controlled exposure levels.
Growth prospects tie tightly to the bio-based and green chemistry movement. With renewable feedstocks gaining traction, methyl lactate could play an even larger role as both solvent and intermediate in sectors under pressure to decarbonize and cut hazardous waste. Expansion in pharmaceuticals, advanced materials, and even food packaging will likely follow from continued refinement in purification and scale-up. Companies experimenting with circular economy models recognize the value of biodegradable intermediates like methyl lactate. As more firms adapt their chemistry to “fit” tighter environmental and safety regulations, methyl lactate’s reputation as a practical, lower-risk solution should only strengthen, particularly if cost and supply can keep pace with demand.
Methyl lactate doesn’t sound like something you’d want to keep under the kitchen sink, but it shows up in more places than you might think. Born from the marriage of lactic acid, made by fermenting sugars from cornstarch or beets, and a touch of methanol, its roots reach into renewable agriculture, not smokestacks and oil rigs. We need more green chemistry like this in our world. Living through countless debates about pollution, petroleum, and the messes left behind by traditional solvents, I’ve seen why it matters to reach for safer choices. Companies turn to methyl lactate as an alternative to petroleum-based cleaning agents, simply because it comes with less worry about toxicity and persistence in the environment.
I remember working in a small manufacturer’s back office, watching employees struggle with harsh degreasers loaded with warning stickers. Methyl lactate found its way into that space not by choice, but out of necessity—people got tired of the eye-watering fumes. Since it’s a good solvent but much milder on the nose and skin, it swapped places with some of the nastier chemicals in the degreasing stations. Big chains pitch it for industries like electronics and auto-parts cleaning. It can break down oils and greases just like the heavy-duty stuff, but the difference is it won’t kick up as many health complaints or leave behind stubborn residues.
Dig into a list of lotion or nail polish remover ingredients and methyl lactate pops up more often these days. It helps blend components together and gives beauty products their smooth feel without that aggressive solvent smell. The personal care industry dances around regulations about what’s safe to put on the skin, so falling back on a plant-based, less volatile option feels like an easy call. Cosmetic chemists look for ways to keep formulas performing well while staying in the clear with regulators. Ingredients like methyl lactate give brands confidence they’re not trading consumer safety for product stability.
People ask why methyl lactate gets chosen over “old faithfuls” like acetone or toluene. The answer seems clear—folks just want to breathe easier and work with less risk. The world doesn’t stop needing strong cleaners, but there’s not much nostalgia for burning eyes and heavy warnings. Researchers point out that it readily biodegrades, which lines up with the push in many cities to phase out “forever chemicals.” That doesn’t mean it’s entirely harmless—good lab safety and proper handling still matter. Dosing up a workplace with any solvent without ventilation doesn’t serve anyone well.
Finding solvents that work without consequences feels like a never-ending test. Methyl lactate checks many boxes, but it arrives with a higher price tag than some traditional options. Some manufacturers hold off on switching because budgets control every decision. Years of working in industries clinging to old standards taught me that price, not just performance, shapes the chemical supply chain. Maybe bigger adoption and smarter production techniques can bring costs down, making it easier for all businesses—big and small—to say yes to safer, plant-based alternatives like methyl lactate. For communities worried about air and water contamination from industrial solvents, every bit of progress counts.
Methyl lactate comes from lactic acid, which you find in sour milk and even inside your muscles after a good workout. Chemists make methyl lactate by adding a bit of methanol to lactic acid. It’s a colorless liquid with a faint sweet smell. In the cosmetics aisle, you see it as a solvent and mild exfoliator, showing up in skin creams, nail polish removers, and facial cleansers.
Many people want products that feel gentle and don’t harm skin. If you've ever picked through ingredient lists because your skin throws a fit over unfamiliar chemicals, you’re not alone. Brands often market formulas as “clean” or “safe,” but that call depends a lot on the actual science.
Research on methyl lactate offers some reassurance. The Cosmetic Ingredient Review (CIR) Expert Panel looked at the available studies and found little reason to worry at common concentrations—usually around 1% or less. Typical risks pop up with pure solvents in large amounts, but cosmetics use much less.
Regulatory agencies across the United States and Europe haven’t flagged methyl lactate as unsafe, although wearing sunscreen or lotion doesn’t mean you’ve signed up for a chemistry experiment. Reports of skin irritation or allergic reaction stay rare. Personal experience and trusted dermatologists confirm that—unless the product sits on broken skin or sensitive areas—most people don’t notice any reaction.
I spent some time working behind a cosmetics counter, and customers wanted formulas that spread evenly or washed off without a mess. Methyl lactate helps dissolve sticky ingredients and keeps creams smooth. It also offers a gentle exfoliating effect, much lighter than heavy-duty acids, which works for people looking for day-to-day skin maintenance.
Its cooling sensation grabs attention. During peak summer, lotions and gels with methyl lactate feel refreshing without stinging. It’s not there to be fancy—it has a job, and most formulas add just enough to get the clean-up or cooling done without tipping things into irritation territory.
Of course, some folks prefer to stick with ingredients they can easily pronounce or trace to plants. Concerns over build-up of synthetic chemicals are real for some, and I get it—my own family has dealt with eczema and allergies, making every cosmetic and soap switch a cautious event. At the same time, methyl lactate breaks down into lactic acid and methanol, both of which clear out quickly in small amounts.
Environmental worries linger, since ingredient manufacturing sometimes leaves behind chemical waste. Greater demand for transparency means companies ought to track sourcing and chemical byproducts. With new research coming out all the time, it’s smart for scientists to keep an eye out for data on long-term use, especially as the clean beauty trend grows.
Safety depends on concentration, skin type, and how often you use the product. Even water or essential oils cause irritation at the wrong strength or application. For methyl lactate, patch testing when trying a new cosmetic gives peace of mind, especially if you have sensitive skin or a history of allergies. Keeping ingredients simple—and asking manufacturers for third-party safety studies—often works best for picky or damaged skin.
Dermatologists and scientists alike recommend checking for regulatory labeling: a product made in the U.S. or Europe usually faces strict oversight, which limits unsafe ingredients. Knowledge isn’t about fear. Knowing what goes on your skin helps you make choices that fit, instead of just grabbing a bottle because it’s trending.
Methyl lactate doesn’t show up everywhere in daily life, but it’s made a solid name for itself in industries like cleaning and flavor production. This compound exists as a colorless liquid at room temperature. Its faint, slightly sweet scent often reminds people of its roots—derived from lactic acid and methanol. People working with this chemical notice its density sits just below that of water, around 1.07 g/cm³, and that makes handling it straightforward. The boiling point likes to stay around 145°C. It’s handy to remember that in a warm climate or heated process, this chemical moves into vapor form faster than you might expect.
In real-world settings, methyl lactate favours mixing with water and many organic solvents like ethanol and acetone. This property comes in handy—cleanup crews, lab folks, and equipment operators benefit from a solvent that washes away easily and leaves less mess. That water affinity helps reduce some storage risks, but it still calls for a tight lid and cool storage since methyl lactate evaporates faster than water. Anyone who’s handled volatile solvents knows keeping containers snug and spill-proof beats mopping up slick floors and worrying about fumes.
On the chemistry side, methyl lactate falls under the ester family. That means it tends to react with strong acids, bases, and water—especially under heat or in the presence of catalysts. In practical terms, this reactivity drives its popularity in green chemistry projects and the manufacturing of biodegradable solvents. For flavor or fragrance industries, its mild nature means less irritation and smoother application.
Speaking from shop-floor experience, spills happen, and knowing a solvent won’t eat through gloves or pipes speeds up cleanup and reduces equipment breakdown. That’s a detail every safety-conscious crew appreciates. Methyl lactate also breaks down into lactic acid and methanol under hydrolysis. Both byproducts are well-studied, bringing fewer regulatory headaches than some stronger, nastier solvents.
Field reports show methyl lactate earns an “easy-to-work-with” badge compared to many other industrial solvents. Direct skin or eye contact still causes mild irritation, so basic safety gear remains a must. Its low acute toxicity gives people peace of mind, though no one wants to breathe in mist or fumes for any length of time. Ventilation—natural or forced—makes the shop floor, lab, or flavor workshop more comfortable.
Waste disposal often causes headaches in manufacturing, but methyl lactate breaks into compounds that meet tighter environmental standards. Local water boards and workplace safety agencies point to this trait as a win over legacy chemicals like acetone, toluene, or heavier esters. Safer solvents mean less paperwork and happier crews.
Industries searching for sustainable options give methyl lactate another look for its greener profile. In my experience with cleaner production campaigns, switching to this solvent can shave off VOC emissions. It still calls for responsible use—spills near water supplies or drains don’t get a free pass, but risks are smaller than with the tough, older solvents. Crews respect a chemical that does its job without leaving a lasting footprint.
People want safer, sustainable materials in their homes and workplaces. Methyl lactate addresses that demand through its physical and chemical abilities, not just theoretical potential. Years of hands-on handling, cleanup, and process improvement show when new options like this work—fewer headaches, simpler safety measures, and smoother production flows.
Methyl lactate often finds its way into labs and manufacturing floors thanks to its role in producing solvents, flavoring agents, and cleaning products. My own work in a research lab brought me face-to-face with this chemical more than once; each experience underlined how easy it is to overlook storage basics in favor of convenience. Taking shortcuts just invites unnecessary risk, especially with substances that can catch fire or cause irritation. Safety isn’t just a checkbox on a form. It shows respect for coworkers, property, and the planet.
For most liquid chemicals, there's a temptation to store things wherever space pops up. That habit quickly leads to trouble. Methyl lactate doesn’t explode under normal conditions, but it’s still flammable. Setting the bottle right next to a heat source or sunlight is just asking for issues. Even a minor temperature spike from a nearby steam pipe or a sunbeam through a window can make it risky.
The best spot for methyl lactate remains a cool, well-ventilated room away from direct sunlight. A flammables cabinet gives extra peace of mind, especially in busy labs or workshops where people juggle open flames or electrical equipment. Storing chemicals on the floor looks convenient but almost always leads to damaged bottles. I learned early in my career never to stash containers near doors or passageways, because getting knocked over remains one of the quickest ways to become “the story” at the staff meeting.
Labels help, but good habits do even more. Pouring methyl lactate without gloves, thinking “it’s just a small amount,” tends to end badly. It can irritate skin or eyes, and inhaling it every day exposes workers to chronic harm. Not every reaction shows up right away, but headaches, dryness, and long-term respiratory effects build up over time.
Carrying methyl lactate in sealed, labeled containers makes retrieval and transport less stressful. Strong, shatter-resistant bottles leave a lot less room for regret. Using spill-control trays and simple absorbent pads helps contain drips or leaks, even on the best-run teams. Forgetting to check for cracked stoppers or rusted lids often leads to mystery puddles—clearly labeled warning signs help everyone avoid accidental contact. I once watched an entire shift stop while someone searched for a missing hazard sticker: better to double-check labels than hold up a project.
Soak up spills right away with absorbent material, and never use water to dilute a big mess. Too much water can actually spread the chemical further, making a small spill into a big problem. Ventilating the area keeps fumes from building up. Waste disposal should always follow local hazardous waste rules—pouring leftovers down the sink only looks easy until regulators show up.
Supervisors should regularly review chemical storage, not just during inspections. I once saw a small reminder board near the storage shelves, listing key points for each chemical—simple, but people remembered the rules. Training every new hire, revisiting protocols at team meetings, and using safety data sheets for reference all help keep the workplace safe. Storing and handling methyl lactate isn’t just a chore—it shows everyone’s commitment to health, safety, and quality.
Methyl lactate has built a reputation as a safer option compared to harsh industrial solvents, earning fans among labs and cleaning products. Coming from renewable resources like corn or sugar beets, it steps away from petroleum-based routes that dominate many chemicals today. That renewable tag brings hope for those hunting greener options. On paper, it looks a lot kinder to the climate than the likes of toluene, acetone, or xylene, which all rely on fossil fuels.
People ask if methyl lactate actually breaks down in nature. Studies show microbes have no trouble eating up methyl lactate under both aerobic and anaerobic conditions, turning it into water and carbon dioxide in weeks or months under normal soil or wastewater environments. There is research from European regulatory bodies showing more than 70% of methyl lactate vanishes within 28 days in standard biodegradability tests. I’ve seen this echoed in environmental health discussion—wastewater plants treat it just like lactic acid or other natural esters. That means accidental spills or rinse water from a cleaning process won’t keep polluting long term. This points to a drop in lingering residues, compared with heavy-duty solvents that persist for years.
Methyl lactate also wins points for health risks. Traditional cleaning agents can irritate the skin or lungs, or cause big headaches when they evaporate. The mild, almost sweet smell is not only easier to handle, but several toxicology studies recognize methyl lactate's low toxicity. No chemical is risk-free, though. High exposures could cause mild irritation. That said, it’s gentler on workers, and there’s less fuss about special containment for routine cleaning jobs.
Making any chemical product means digging into more than just where it ends up. People often forget to ask about what it takes to make it in the first place. To create methyl lactate, plants like corn get grown, harvested, and refined. That soaks up land, water, and fertilizer. Factories then ferment the sugars, turn them into lactic acid, and finally make methyl lactate through chemical processing. Compared to fossil processes, the process cuts down on greenhouse gas emissions—if the agriculture side doesn’t overuse pesticides or fossil-fueled machinery.
There’s another catch. Methyl lactate remains flammable, so transport and handling regulations can still trip people up. Spills in waterways would break down fairly quickly, but overuse or dumping large amounts in fragile habitats might still stress ecosystems before microbes catch up.
Paints, inks, and cleaning companies have swapped in methyl lactate not only for the environmental story but also because tighter rules push out many traditional solvents. In my own work trying to clean stubborn ink stains, methyl lactate-based removers rarely require masks or open windows the way older products do. They still boast strong cleaning power, just without as much environmental headache.
Plenty of labs and companies track how much methyl lactate they use and watch for any new research. They invest time in staff training and disposal guidelines, aiming to keep as much of this material recycled or properly treated. Regulatory frameworks already flag persistent organic pollutants for phase-out, but methyl lactate usually doesn’t linger long enough to raise those alarms. In summary, people scan more than just biodegradability—energy use, farming impact, and overall safety all build the full story.
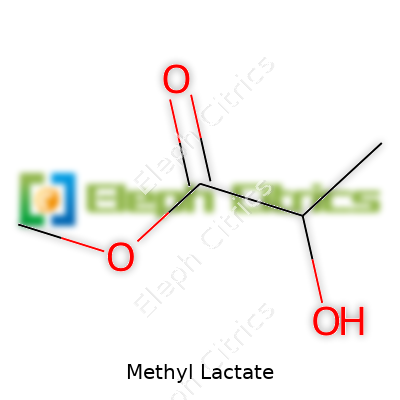
| Names | |
| Preferred IUPAC name | Methyl 2-hydroxypropanoate |
| Other names |
Lactic acid methyl ester Methyl 2-hydroxypropanoate Methyl α-hydroxypropionate Methyl lactate ester |
| Pronunciation | /ˈmɛθ.ɪl ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 547-64-8 |
| Beilstein Reference | 'Beilstein Reference 1723209' |
| ChEBI | CHEBI:17816 |
| ChEMBL | CHEMBL3301012 |
| ChemSpider | 54618 |
| DrugBank | DB11262 |
| ECHA InfoCard | echa infocard 100.009.073 |
| EC Number | 202-220-2 |
| Gmelin Reference | 8337 |
| KEGG | C02685 |
| MeSH | D008765 |
| PubChem CID | 6114 |
| RTECS number | OD9625000 |
| UNII | 26N5N32W13 |
| UN number | UN3278 |
| CompTox Dashboard (EPA) | DTXSID2044364 |
| Properties | |
| Chemical formula | C4H8O3 |
| Molar mass | 104.10 g/mol |
| Appearance | Clear, colorless liquid |
| Odor | faint pleasant odor |
| Density | 1.092 g/cm³ (at 20 °C) |
| Solubility in water | Miscible |
| log P | -0.18 |
| Vapor pressure | 0.14 mmHg (20°C) |
| Acidity (pKa) | 15.1 |
| Basicity (pKb) | 15.1 |
| Magnetic susceptibility (χ) | -7.53×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.4200 |
| Viscosity | 2.5 cP (25°C) |
| Dipole moment | 2.53 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 158.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -682.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | −1195.5 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A01AD11 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P271, P280, P302+P352, P305+P351+P338, P312, P337+P313, P362+P364 |
| Flash point | 120 °C |
| Autoignition temperature | 225 °C (437 °F; 498 K) |
| Explosive limits | Explosive limits: 2.6–12.6% (in air) |
| Lethal dose or concentration | LD50 Rat oral 2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 2,600 mg/kg |
| NIOSH | WI1140000 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Methyl Lactate: Not established |
| REL (Recommended) | Rel=0.5 |
| Related compounds | |
| Related compounds |
Lactic acid Ethyl lactate Propylene glycol Methyl acetate Methyl pyruvate |