Over the last century, the world scientific community has explored metal-organic compounds for their unique uses. Molybdenum citrate emerged from these experiments as researchers looked to pair transitional metals with biocompatible ligands. In the early years, the focus sat squarely on basic coordination chemistry. By the 1960s, the growing understanding of both molybdenum and citric acid created opportunities to experiment with new compounds in agriculture and medicine. As industry and academic labs dug into the potential of chelated metals, molybdenum citrate found its place as a promising supplement and reagent. Developments across Europe and North America expanded its role. These pioneers got their hands dirty testing synthesis methods, watching stability, and trying to pin down just what set this compound apart from other molybdenum salts.
Molybdenum citrate combines molybdenum, a silvery transition metal, with citric acid, familiar for providing tartness in lemons. This compound generally forms as a green or pale yellow powder, crystalline to touch and stable under dry conditions. It mixes water solubility, low toxicity, and chemical versatility, which keeps it valuable for feeding trace elements to plants, crafting certain catalysts, and helping with lab-scale reactions. Lab-grade batches follow purity standards tightly, with reputable sources listing detailed breakdowns. Many commercial suppliers sell it for research use only, and buyers in agriculture or chemical processing know to check purity and impurity levels up front before using it.
A closer look at molybdenum citrate’s physical makeup shows a molecular weight that lands near 476 g/mol, though the exact value moves with hydration and formulation. Pure samples carry a mild odor that some researchers liken to very mild metal shavings, mixed with citric acid’s faint aromatic tang. As a water-soluble compound, molybdenum citrate forms clear to slightly cloudy solutions. In lab and field tests, it resists decomposition under standard storage. Chemically, this compound offers a reliable source of molybdenum in various oxidation states—notably Mo(IV) and Mo(VI)—which makes it valuable in redox chemistry and as a micronutrient source for certain plants. Its solubility in water beats that of elemental molybdenum or molybdate salts, helping with mixing and dosing in agricultural and research contexts. The pH of a 1% solution usually sits between 3.5 and 5.5, so it solves without significant shifts in acidity for most soil and solution types.
Any chemical product aims to back up its claims with data, and molybdenum citrate is no exception. Suppliers must indicate batch-specific data on purity, typically ranging from 97% to 99% for research chemicals, with labeling required to note key impurities like heavy metals (lead, arsenic, cadmium). Standard labeling also demands CAS number, molecular formula (often C6H5MoO7 or similar, according to hydration state), as well as lot number and manufacturer information for traceability. Most containers fall under UN-approved packaging rules when shipped internationally, and hazard symbols—though not always required—must follow the Global Harmonized System. Customers in food or agriculture need certifications proving compliance with local safety rules, and every bottle or drum carries storage advice: “Store in a cool, dry place. Protect from moisture.”
Getting molybdenum citrate takes some patience and know-how. Most suppliers rely on direct reaction of sodium molybdate (Na2MoO4) with citric acid in aqueous solution. The reaction is stirred at controlled temperature, usually kept slightly warm—that helps dissolve citric acid and molybdate fully. The mixture then settles or gets filtered, with final evaporation or precipitation steps drawing out the molybdenum citrate solid. Commercial labs use vacuum dryers or rotary evaporators, taking care not to overheat, which could break down the delicate chelate bond. Operators follow strict routines for wash steps that remove sodium and free acid. Larger-scale producers have automated much of this, but fundamentals stay the same: keep it clean, use good reagents, and watch for telltale changes in color that signal the reaction’s progress. Small-batch chemists swap stories about the best volume ratios to get the cleanest crystals with minimal leftover citric acid.
Molybdenum citrate may look mild, but it takes part in a surprising range of chemical transformations. In water, it can release molybdenum ions gradually, useful for slow-release micronutrient formulas in hydroponics and soil treatments. Researchers also use it as a reducing agent or oxygen transfer reagent in bench chemistry, as its molybdenum centers switch states with relative ease in the right setup. Complexes formed with additional ligands, like amines or phosphines, allow fine-tuning for use in catalysis or as model bioinorganic compounds. This adaptability keeps molybdenum citrate on the workbench for organic synthesis and analytical chemistry. Newer studies use its reactivity for building nanostructured materials, where citrate both templates and stabilizes growing metal clusters.
Across supplier catalogs, molybdenum citrate pops up under a handful of aliases. Molybdenum(IV) citrate, trisodium molybdenum citrate, and simply “citrate complex of molybdenum” show up most often. The differences usually follow from exact formulation—some products include sodium, others match higher or lower oxidation states. Patent filings and agricultural supply sheets sometimes call it “Mo-citrate nutrient” or “fertilizer-grade molybdenum citrate.” Food chemistry research prefers “molybdenum-citrate chelate.” Each name covers the basic structure: a citrate ion (C6H5O7) wrapping around molybdenum, usually with a few water molecules hanging on. Careful sourcing means checking label details, not relying on the name alone, since impurity profiles and hydration matter in some uses.
Handling molybdenum citrate in the lab, on the farm, or during transport, safety takes priority. OSHA recognises molybdenum compounds as materials to respect but not to fear. Chronic exposure above recommended levels can harm metabolism, so operators wear gloves and safety glasses, especially around powder handling or solution preparation. Inhalation avoidance remains standard. Waste collection follows local hazardous material rules—no dumping in regular trash or down lab sinks. Material Safety Data Sheets point to oral and respiratory exposure thresholds, reminding users that molybdenum, even as a micronutrient, can build up in animal and human systems. Agricultural workers look at recommended ppm (parts per million) rates for field applications, keeping in mind the delicate balance between deficiency and toxicity in crops.
In agriculture, molybdenum citrate plays a key role as a micronutrient supplement, supporting nitrogen fixation in legumes and boosting efficiency in certain fertilizers. Soil scientists have used it to treat deficiencies that lead to stunted growth—precisely because citrate complexes let plants take up molybdenum more easily. In chemical research, it helps as a source of molybdenum ions in analytical testing and synthesis experiments, especially where stable but reactive forms are needed. A smaller—but crucial—demand comes from the catalysis sector, where molybdenum citrate serves as a precursor to heterogeneous or supported catalysts for chemical manufacturing. Water treatment, electronics, and material science researchers have found use for it in thin films, nanomaterials, and surface coatings. Food and animal feed producers add tightly regulated, low-dose molybdenum citrate for dietary supplementation, supporting ruminant health and productivity under veterinary oversight.
Laboratories across the globe stay busy probing the next chapter of molybdenum citrate’s story. Most recent trends look at improved solubility, new synthetic methods, and the use of test-tube-grown molybdenum citrate nanoparticles in environmental catalysis. Computational chemists map electronic transitions, helping to guide new uses in clean chemistry and energy storage. Agronomy research investigates blends of molybdenum citrate with phosphates, aiming to boost root uptake and efficiency in depleted soils. Pharmaceutical experiments occasionally trial molybdenum citrate’s antioxidant properties. Each development cycle brings up questions about purity, storage life, and environmental impact. Collaboration among chemists, biologists, and agronomists has driven faster testing, with journals now filled with reports on stability, compatibility with various additives, and the mechanisms behind improved biological absorption.
Molybdenum citrate, while safer than many heavy metal salts, still demands respect. The element molybdenum itself is essential for plant and animal life, but excess causes problems ranging from crop discoloration to chronic metabolic issues in cattle. Toxicity studies from North America and Europe plot dose-response curves in animals, matching real field conditions. Recent public health data show that carefully managed supplementation in food and agriculture avoids toxicity, keeping animal tissues and human foods in the safe zone. In vitro experiments dig into molecular pathways, looking for oxidative stress or DNA damage at high concentrations. So far, controlled dosing shows little risk, but the margin narrows if accidental overuse happens. Agricultural extension offices remind farmers and feed producers to stick to recommended rates, especially where soil molybdenum runs high.
Looking down the road, molybdenum citrate’s prospects feel closely tied to global agriculture, clean energy, and specialty chemistry. Crop scientists predict more demand in sustainable farming, as efficient micronutrient supplements become a tool for food security on depleted land. Chemical engineers plot out its use as a source compound for greener catalysts and high-performance batteries. Material scientists eye the role of citrate-chelated molybdenum in nanotechnology, especially in water purification and coatings with antibacterial or self-cleaning properties. Regulatory bodies keep an eye on purity and labeling, aiming to keep adulterated supplies out of food and agriculture chains. On the academic front, molybdenum citrate’s mix of safety, versatility, and reactivity promises more funding and wider international collaborations. On a practical level, it sits ready to show its worth as long as users keep a clear handle on dosage, sourcing, and best-fit applications for their needs.
People rarely talk about molybdenum, but it’s quietly crucial in many parts of modern life. Molybdenum citrate is not your everyday supplement or ingredient, yet it plays a meaningful part both in biology and in a handful of specialized industries. Looking around, the majority of us miss how these minerals touch our lives, but digging deeper shows molybdenum citrate’s value isn’t a stretch of the imagination.
Soil can make or break a harvest. Farmers trying to grow beans, lentils, or other legumes deal with one challenge above all—how well their plants fix nitrogen. This invisible trick happens because bacteria in the roots break down nitrogen in the air. Molybdenum citrate gets used as a micronutrient to help crops like soybeans and peas carry out this process. Without enough, the enzyme nitrogenase loses its punch, and farmers see less yield. Researchers out of Iowa State have shown better grain output when soils are balanced with enough molybdenum. From the ground up, many signs of healthy crops link back to these tiny mineral boosts.
Molybdenum counts as an essential trace mineral. The human body uses it to build enzymes that handle sulfur-containing amino acids and toxins. Without enough, the body struggles with breaking down leftovers in food and medicines. Most folks get enough through common foods like grains, beans, and peas. But in rare medical settings—maybe with a genetic disorder that slows molybdenum absorption—doctors use compounds like molybdenum citrate to help balance things out. Scientific studies, including research in the American Journal of Clinical Nutrition, recognize its importance for normal enzyme activity.
Chemists in the lab value molybdenum citrate for more than its biological role. It shows up in catalyst design, helping reactions along that otherwise slug through slowly. Catalysts containing molybdenum often drive steps in oil refining, plastics, and pharmaceutical manufacturing. The citrate form makes blending and handling easier because it’s water soluble. Many companies value easy-to-use compounds to keep lab work and industry running smoothly. Teaming up with copper, sulfur, or iron, molybdenum sits at the crossroad of many new catalyst designs meant to save energy and reduce waste.
Everything with potential can present a risk. Too much molybdenum, even in the citrate form, can pose health concerns. Health authorities like the National Institutes of Health warn against high supplemental doses, as overconsumption can hurt metabolism or create copper deficiency. Environmentalists also follow how much molybdenum runs off from fertilizers or industrial plants, as excess can damage aquatic life. Monitoring, careful application, and following what scientists learn all matter for safety.
Molybdenum citrate’s value tends to stay behind the scenes, working quietly in the soil, the lab, or the clinic. Farmers, chemists, and doctors build on what research shows about this element. Keeping an eye on its use—avoiding overdoses and keeping environmental health strong—stands out as the wise path. In this world where minerals often get overlooked, molybdenum citrate keeps showing people just how big a difference a small compound can make.
Our bodies need minerals. That’s not some old-fashioned advice from the doctor, but the truth we all live with. Molybdenum, though, often gets left out of the conversation. Still, this trace mineral keeps us running, even if most never talk about it. Molybdenum citrate steps in as a supplement for anyone struggling to meet their dietary needs from food alone.
I started paying attention to molybdenum when reading up on metabolic health—not something most folks spend lazy Sundays doing, but my family’s history with gout and uric acid issues got me looking for answers. The more I dug in, the clearer it got: molybdenum runs enzymes that break down sulfites and drugs. It’s especially key for the body’s process of turning sulfites—preservatives in things like wine and dried fruit—into safer substances we can pass out through urine. Without enough, people may experience headaches, allergic reactions, or long-term buildup of harmful products.
People who eat mostly processed foods, or those with digestive problems, sometimes face a shortage. In rare cases, genetics can curb one’s natural supply. Supplementing with molybdenum citrate helps bridge that gap, letting those key enzymes do their job.
Enzyme Health Molybdenum acts as a core piece in enzymes like sulfite oxidase, xanthine oxidase, aldehyde oxidase, and mitochondrial amidoxime reducing component (mARC). These enzymes clear out waste, balance nitrogen, break down drugs, and turn food into energy. Anyone with recurring sensitivity to foods with sulfites, or who struggles breaking down medications, may find genuine help from adequate molybdenum.
Detoxification Every year, more folks talk about detox. It has become a buzzword in wellness circles, but the real science sits in minerals like molybdenum. In my own circle, people sensitive to preservatives have noticed fewer headaches after supplementing. The effect isn’t magic—it’s a sign their body finally processes those chemicals.
Supporting Uric Acid Breakdown A diet rich in meats and certain fish brings uric acid problems for people like me. Molybdenum’s job in the enzyme xanthine oxidase lowers purine buildup, meaning fewer gout flare-ups. Studies published in nutritional journals point out this connection isn’t just theory.
Most supplements have risks if overdosed. Molybdenum is no exception. Too much may bring on copper deficiency and joint pain. For most adults, 45 micrograms per day cover what’s needed. The upper limit, according to the National Institutes of Health, sits at 2,000 micrograms. In my routine, I check food labels, choosing grains, legumes, and nuts, and only add a small tablet when the diet falls short.
Anyone dealing with kidney disease, copper imbalance, or rare metabolic problems should talk to a health professional first. Good advice from a doctor or registered dietitian wins over online opinions any day.
People rarely see the silent work of minerals, but it shows up in energy, sleep, and how we handle stress or allergens. The benefits of molybdenum citrate carry weight for those with limited diets, increased preservative exposure, or family histories of metabolic challenges. With proper use, it works as quiet support for the body’s built-in cleanup crew, helping us feel better without a second thought. Keeping a close eye on diet and supplementing wisely makes more of a difference than chasing trends. That’s a habit worth keeping.
Molybdenum often sounds like one of those minerals you only hear about in science class, not something tied to our daily lives. Yet, it plays a pretty real role in keeping our bodies running. Often tucked into supplements and sometimes combined as molybdenum citrate, it helps enzymes break down toxins and supports the metabolism of sulfur-containing amino acids. Most of us get enough molybdenum from grains, legumes, and leafy veggies, so supplementing usually isn’t needed unless there’s a deficiency or a doctor gives the nod.
Tossing any new supplement into your routine can feel risky, especially with health headlines always warning of hidden dangers. Molybdenum citrate seems pretty safe for most adults at typical doses—think less than 2,000 micrograms per day, according to the National Institutes of Health. The body flushes out excess molybdenum through urine, so it usually doesn’t stick around to cause problems. True side effects at low doses have been rare in medical studies and case reports.
Trouble only starts when people keep piling on high doses over months or years. Too much molybdenum can irritate joints, causing symptoms that mimic gout, like swelling and pain. Some studies have shown elevated levels may nudge up uric acid—a trigger for those familiar with gout flare-ups. High intake for a long time could possibly mess with copper absorption, which my grandma’s old farmer health books always warned about, leading to fatigue, anemia, or even neurological problems. These effects come from chronic, heavy exposure—think folks working with mining dust or industrial chemicals, not a multivitamin.
Healthy adults grabbing the occasional supplement don’t usually run into big risk. Folks with existing kidney issues or rare genetic metabolic conditions would be smart to avoid extra molybdenum, as their bodies already struggle to clear out the mineral. Parents tempted to give kids these supplements should pause, since children need far less and their “safe” limit is several times lower than adults’. Pregnant or breastfeeding people ought to lean on doctor guidance, because there’s less data available for these groups.
Overuse crops up when people start self-diagnosing based on internet trends. Some “wellness” corners hype up mineral blends for energy, detoxing, or metabolic boosts without good evidence. Buyers often don’t realize more is not better—especially since regulations on supplement labels and purity are spotty in many countries. It’s happened before with zinc and selenium, where folks overshot the mark chasing immune benefits.
One approach would be more honest labeling and tighter oversight, so companies have to prove their claims. Benefits and risks might need to stand out clearer, instead of being buried in fine print. Doctors and dietitians can also take a more direct role—discussing supplement use during checkups, so patients don’t end up risking their health over a wellness fad.
Real insurance for avoiding trouble is a balanced plate, not a handful of pills. People with clear medical advice for extra molybdenum citrate should follow the guidance. For everyone else, variety in foods usually covers any need. Whenever uncertainty creeps in, I still find that asking a medical pro keeps things in check far better than guessing with new bottles off the shelf.
Molybdenum isn’t a word most folks toss around, but it’s a mineral found in foods like beans, lentils, nuts, and even leafy greens. The body uses it to help kick-start certain enzymes that break down amino acids and waste, especially in the liver and kidneys. Sometimes, people think about adding a supplement if their diet falls short or if a doctor spots a possible deficiency.
The first step to taking any supplement—molybdenum included—should be talking to a health professional. I’ve learned that even minerals from natural sources can cause problems if the balance tips too far in one direction. Most adults don’t face molybdenum deficiency because a typical diet covers the recommended intake. So before grabbing a bottle from the shelf, getting medical input helps avoid overdoing it and risking toxicity.
I remember trying a supplement on my own once, thinking more equals better. It didn’t take long to learn that minerals don’t quite work that way. The recommended dietary allowance for adults is pretty low—about 45 micrograms per day, according to the National Institutes of Health. High doses, such as several milligrams daily, can be harmful over time. Some people experience joint pain, gout-like symptoms, and digestive problems after absorbing too much. It’s wise to check the label and not assume every capsule matches what you need.
Molybdenum citrate comes in tablet and capsule form. Taking it with a glass of water during a meal often helps the body absorb it and minimizes any risk of stomach upset. It’s also easier to remember a routine if it lines up with breakfast or another daily habit. Sticking to a set time helps avoid missed doses, especially with small minerals that don’t offer instant feedback like caffeine or vitamin C.
Supplements can sometimes clash with medications or other nutrients. For example, copper and molybdenum compete inside the body; too much molybdenum can drop copper levels. Some studies show that long-term use can even fuel unwanted interactions in people already taking medicine for gout. Bringing a list of your current supplements and prescriptions to your next medical visit ensures nobody’s guessing about risks.
With so many brands out there, picking a reliable product matters. I always check whether a supplement has been tested by third-party organizations like NSF International or USP. These badges tell me the product’s contents match the label, without unwanted extras. Sticking to reputable companies helps avoid the worst confusion that comes with unfamiliar pills off the internet.
Ultimately, supplements shouldn’t replace what a balanced meal provides. Tracking what you eat for a few days with an app or food journal might show you’re already getting what you need. For people with certain medical conditions or absorption issues, supplementing works best under medical supervision. An annual checkup and a smart conversation with a professional go farther than any guesswork at the vitamin aisle. Responsible decisions today keep tomorrow free from unexpected surprises.
Molybdenum forms part of the story for people who want stronger health from supplements. It’s an essential trace mineral. Your body depends on it to support enzymes and help break down amino acids and toxins. The citrate form, sometimes chosen for its improved absorption, crops up in many daily vitamins. The question about long-term safety gets asked more as people look for the next boost to vitality or disease prevention.
Most people pick up enough molybdenum from a balanced diet – think nuts, beans, grains, and leafy greens. Average daily needs run low, less than 50 micrograms for adults, and the body doesn’t store excess. The kidneys flush out what’s not needed. Concerns grow when daily intake jumps because of supplements, often stacked on top of already adequate diets.
Health agencies put the upper intake level for molybdenum at about 2,000 micrograms per day for adults. That’s a massive gap between what most folks get and what’s flagged as risky, but that doesn’t make very high intake harmless. The rare poisonings described in medical cases almost always involved heavy industrial exposure, not supplements. Even so, too much can throw off copper balance, leading to joint pain, gout-like symptoms, or other mineral imbalances. I’ve seen people double down on a handful of supplements without realizing the compound effect, a risk that invites subtle problems over time.
There isn’t a ton of research teasing out the effects of taking molybdenum citrate every day for years. Most studies run short, usually a few months, often on small groups. Data does point to low toxicity at usual dietary levels, and even most supplement doses shake out as safe in the short term. Long-haul use, especially in folks who already eat plenty of plant foods, doesn’t get much coverage in clinical journals. Medical professionals caution against chronic high dosing for this reason – unknowns linger, especially for people with kidney issues, as the ability to flush minerals can drop off sharply with age or disease.
Not everyone needs more molybdenum. Vegans and vegetarians probably hit the daily requirement anyway. People with rare genetic metabolic issues, like sulfite oxidase deficiency, may be prescribed molybdenum, but that’s under strict care. For most of us, the real risk comes from indiscriminate supplement stacking. It’s easy to miss overlap between multivitamins, fortified foods, and specialty blends. Older adults and those with kidney disease face steeper risks, simply because their bodies don’t clear extras as efficiently.
Lab testing helps spot unusual mineral levels before problems set in. Talk to your doctor about any new pills, especially if you’re juggling medications or chronic health conditions. Reading supplement labels for actual content (not just marketing claims) makes a difference. Relying on food for micronutrients keeps intake balanced and sidesteps surprises. Supplements can fill a gap but shouldn’t replace a foundation of real food.
Molybdenum citrate in small, daily amounts looks safe for most healthy adults, yet lasting safety data falls short. Overdoing it doesn’t bring better health and might cause more harm than good over time. Getting minerals from meals that combine beans, grains, and greens offers a better way to support enzyme health than gambling on megadoses from a bottle. Personal experience and the latest science both say moderation wins.
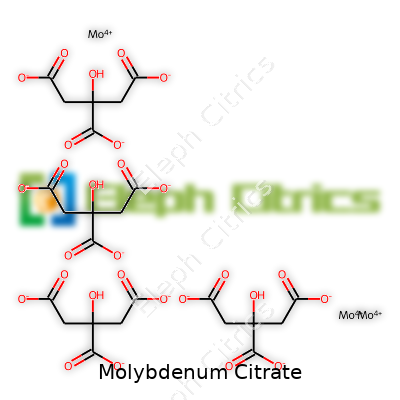
| Names | |
| Preferred IUPAC name | Tris(2-hydroxypropane-1,2,3-tricarboxylato)molybdate(III) |
| Other names |
Citrate de molybdene Molybdenum(II) citrate Molybdic citrate |
| Pronunciation | /məˈlɪb.dɪ.nəm ˈsɪ.treɪt/ |
| Identifiers | |
| CAS Number | [7778-64-5] |
| Beilstein Reference | 18285026 |
| ChEBI | CHEBI:75969 |
| ChEMBL | CHEMBL3306563 |
| ChemSpider | 25204845 |
| DrugBank | DB14542 |
| ECHA InfoCard | 100.040.118 |
| EC Number | 259-504-6 |
| Gmelin Reference | 82884 |
| KEGG | C18586 |
| MeSH | D008983 |
| PubChem CID | 102154276 |
| RTECS number | QA5075000 |
| UNII | 827A17621F |
| UN number | Not assigned |
| CompTox Dashboard (EPA) | DTXSID4087709 |
| Properties | |
| Chemical formula | C6H5MoO7 |
| Molar mass | 976.07 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.55 g/cm3 |
| Solubility in water | Insoluble in water |
| log P | -2.3 |
| Acidity (pKa) | 7.0 |
| Basicity (pKb) | 8.2 |
| Magnetic susceptibility (χ) | +1200e-6 cm³/mol |
| Refractive index (nD) | 1.535 |
| Viscosity | Viscous liquid |
| Dipole moment | 6.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 221.5 J·mol⁻¹·K⁻¹ |
| Hazards | |
| Main hazards | Causes skin irritation. Causes serious eye irritation. May cause respiratory irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07,GHS08 |
| Signal word | Warning |
| Hazard statements | Hazard statements: Harmful if swallowed. Causes skin irritation. Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P264, P270, P271, P273, P280, P301+P312, P304+P340, P305+P351+P338, P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| LD50 (median dose) | LD50 (median dose): Oral (rat): >5,000 mg/kg |
| NIOSH | RN 14265-44-2 |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Molybdenum Citrate: 5 mg/m³ (as Mo, OSHA PEL for soluble compounds) |
| REL (Recommended) | 0.5 mg/m³ |
| IDLH (Immediate danger) | Not Listed |
| Related compounds | |
| Related compounds |
Ammonium molybdate Molybdenum disulfide Molybdenum trioxide Sodium molybdate Molybdenum acetate Molybdenum oxalate |