Nickel citrate represents a chapter in the evolution of transition metal complexes with organic acids. Efforts to better understand how nickel interacts with citric acid date back to early coordination chemistry studies in the 20th century. Chemists were curious about the chelation process because nickel plays an important role not only in industry but also as a micronutrient in biological systems. As industry’s needs grew for more specialized catalysts and metal sources, research groups documented methods for synthesizing and analyzing the properties of nickel-citrate compounds. Over decades, improvements in analytical tools and more refined manufacturing processes brought about greater control in the production and purity of nickel citrate, making it more accessible for a range of laboratory and industrial uses.
Nickel citrate typically appears as a greenish crystalline powder or granule with good solubility in water. Labs and manufacturers use it for a mix of research, catalysis, and sometimes in specialty coatings for electronic parts. Interest in this compound varies by region and industry, but the main draw lies in its ability to deliver both nickel ions and a biocompatible organic ligand in one package. You’ll see it pop up in catalogues from chemical suppliers under various grades, with details tailored to fit academic research, industrial preps, or applied materials science. Its blend of metallic and organic character gives it a versatility other nickel salts can’t always match.
Typical samples show a green color due to the nickel(II) ion. Nickel citrate dissolves readily in water, forming clear green solutions. The compound contains three carboxyl groups from citric acid chelating a central nickel atom. Depending on synthesis conditions, hydrates may form, changing the appearance and water content. It usually decomposes above 150°C, breaking down into nickel oxide, water vapor, and carbon dioxide. The presence of citrate makes this salt less likely to cause rapid hydrolysis or precipitation in neutral or mildly acidic solutions, which makes it a dependable candidate for slow nickel ion release or buffer-sensitive applications.
Chemical suppliers list nickel citrate with detailed purity data, typically ranging from 98% to research-grade higher than 99%. Common technical specs include bulk density, moisture content, presence of trace impurities (like iron, copper, lead), and the exact hydration state. Labels list the chemical formula—usually Ni3(C6H5O7)2·nH2O—along with its CAS number and storage recommendations. Companies pay extra attention to consistent batch quality, since small changes in nickel or citrate levels can affect reactivity or downstream processing. For lab users, specs around solubility, UV-Vis absorption maxima, and nickel content by weight prove most critical.
Most manufacturers prepare nickel citrate by mixing aqueous nickel(II) salts, such as nickel sulfate or nickel chloride, with citric acid. Careful pH adjustment with sodium hydroxide or ammonia forms a solution from which the nickel citrate precipitates as the water evaporates or as the mix cools down. The ratio of nickel to citrate and presence of extra base can tilt the reaction toward the desired product and hydration form. After precipitation, the compound is filtered, washed with cold water to remove residual acids and salts, and then vacuum dried. Consistency in water quality and reaction temperature impacts the final crystal structure and solubility.
Nickel citrate serves as a starting point for a range of chemical reactions. It dissolves in water, dissociating to give nickel ions and citrate ligands, which can participate in redox reactions, coordination chemistry, or help solubilize metals in more complex matrices. Heating causes the compound to decompose, leaving behind nickel oxide, a process valuable for producing fine nickel oxides for ceramics or catalysts. In coordination chemistry, the citrate ligand can be swapped out for other chelators, or the nickel can be exchanged through ion exchange resins, opening up access to related metal-organic complexes. Researchers also look at how pH adjustment, different counter-ions, or substituents on the citrate backbone can produce derivatives with tunable reactivity or selective binding properties.
Nickel citrate pops up under several alternative names: trisodium citrate nickel(II) complex, nickelous citrate, and sometimes just nickel(II) citrate. Chemical catalogues might lump it under metal-organic salts or coordination compounds. Some databases list precise formula descriptors, and suppliers often add product codes to simplify ordering. In research papers and patents, you might see shorthand such as Ni-citrate complex or even homegrown abbreviations that center on the number of nickel or citrate ions. It’s important to check the hydration state in any given sample, as it can have a real impact on mass-based calculations.
Nickel compounds bring serious safety concerns. Nickel citrate isn’t the most dangerous form, but it can still provoke skin and respiratory allergies. Chronic exposure to nickel ions connects with increased risks of dermatitis and lung problems. Laboratories and manufacturing setups stick with gloves, goggles, and proper ventilation. Waste solutions containing nickel must be collected and disposed following hazardous waste guidelines in accordance with local and national regulations. Safety data sheets detail the basic hazards, personal protective gear needed, procedures for spills or exposures, and emergency response recommendations. Consistent training makes a difference in preventing accidental exposure, while regular monitoring of airborne or dissolved nickel concentrations keeps workplace risks low.
You’ll find nickel citrate put to work in diverse spaces. In lab research, it’s popular for growing nickel-containing crystals or serving as a source of nickel in biochemical studies about metal ion binding. Plant biologists use it for micronutrient supplementation, since it offers better control over nickel bioavailability versus insoluble sources. In industry, people mix it with other chemicals to develop nickel coatings and catalysts in electronic manufacturing. Some specialty ceramics and pigments rely on nickel citrate for a clean infusion of nickel without halide residues. As battery research ramps up, interest grows in its role as a nickel source for precursor synthesis or controlled crystal engineering. Each field finds its own uses, often building off the basic solubility and controllable release of nickel ions provided by this compound.
Over the last decade, R&D teams have pushed nickel citrate into new territory. With the demand for tailored materials in renewable energy and advanced electronics, researchers look at how this compound contributes to controlled nanoparticle growth or forms effective catalysts. Startups in clean tech explore its use in waste water treatment, seeing how citrate ligands help nickel grab onto contaminants. Biomedical teams review how nickel citrate interacts with proteins or cells, both for its potential risks and as a way to study trace metal metabolism. Meanwhile, process engineers experiment with greener, less energy-intensive ways of preparing high-purity samples, trying to reduce waste streams and reliance on harsh acid/base reagents.
Toxicologists scrutinize nickel citrate because nickel’s biological effects run deep. Short-term exposure in animal studies points to possible irritation and mild toxicity, mostly if inhaled or handled in large volumes. Long-term studies, especially with other nickel salts, link chronic exposure to increased cancer risk and organ toxicity. The chelating action of citrate appears to modulate, but not erase, nickel’s toxicity. Researchers analyze how citrate changes absorption and metabolic pathways in plants and animals, hoping to pin down situations that could lead to bioaccumulation or inadvertent environmental contamination. Stricter workplace regulations and careful product labeling come straight from these toxicity studies.
Nickel citrate looks set for steady demand as research and industry look for ways to use metal-organic salts more thoughtfully. New battery chemistries may call for precise delivery of nickel ions, while pharmaceutical and medical research studies use it to probe metal-protein interactions without excess toxicity. As sustainability pressures kick in, people ask not only about the performance of nickel citrate, but also how to recover, recycle, or substitute it with less hazardous options. Bio-based alternatives and upcycling of industrial by-products get more attention. The market for compounds like nickel citrate will likely lean on its reliability and adaptability, but success will hinge on smart handling of supply chain risks, clear safety communication, and a willingness to keep improving how scientists and manufacturers deliver high-quality material with less environmental baggage.
Nickel citrate finds a pretty unique place in various fields, and not many people outside the chemistry crowd talk about it much. I remember paging through ingredient lists at my first lab tech job, stumbling on this stuff and asking, “Do we really use nickel for anything besides batteries?” Turns out, nickel citrate is a lot more flexible than folks usually think.
Let’s start with what labs do. In research settings, nickel citrate helps scientists experiment with nickel’s effects without the dangerous side effects of raw nickel salts. This means much safer handling for anyone down the production line. You’ll find it used in catalysts—those little chemical workhorses that make reactions happen faster and cleaner. Look at how waste management outfits handle chemical spills: They sometimes need nickel-based catalysts to break pollutants down. Years ago, I toured a wastewater cleanup site where they talked about testing new catalyst blends using, among others, nickel citrate.
If you’ve ever seen jewelry that looks silver but lasts better, there’s a good chance nickel went into it. In decorative and industrial plating, nickel citrate pops up since it dissolves well, allowing for that shiny, even finish. I remember taking apart one of my dad’s old transistor radios—there were wires coated with nickel for a reason. Plating with nickel gives base metals longer life and more resilience in harsh conditions, and using a citrate complex keeps the plating bath stable. This means less mess and better results.
Nickel has earned its reputation in electronics. Nickel citrate gets pulled in when precise nickel dosing matters, such as in creating certain battery materials. Devices demand reliable, pure components. Using nickel citrate, manufacturers avoid some impurities from natural nickel ore. Growing up, my neighbor’s garage doubled as his workshop, and he always talked about how finicky battery chemistry could be. Getting the right metal in the right form made the difference between a battery that works for months and one you toss after a week.
While nickel’s role in the human body isn’t front-page news, trace amounts show up in supplements and food fortification, especially for folks whose diets are missing critical metals. The body only needs tiny doses. With safer nickel salts like nickel citrate, researchers develop ways to avoid toxicity, which means advancements come with fewer risks. Academics keep studying possible health connections, so it’s a field to watch.
Like many compounds, nickel citrate needs respect. Allergies and toxicity are real issues, and workers need to take safety seriously. Regulatory agencies watch nickel outflows, requiring firms to stick to limits. Health and environment always get consideration. I think back on my own training—nobody could grab a chemical container without a full safety lesson first.
With chemical safety and innovation constantly on the radar, nickel citrate stands out as an example of an ingredient that offers reliability in familiar industries and opens up new ideas in manufacturing, energy, and health research. Companies are investing to keep working conditions safe and waste managed. Researchers don’t stop looking for substitutes that might offer the same performance with lower risks. Every step forward in this area draws on teamwork between scientists, regulators, and regular workers. That’s how real progress happens.
Nickel pops up in trace amounts all over our daily lives—vegetables, grains, even our drinking water. Most folks don’t give it much thought. After all, the body only uses tiny amounts. But nickel shows two faces: one as a micronutrient, another as a culprit in allergies and toxicity. That double-sided nature calls for a closer look at compounds like nickel citrate, which packs nickel with citric acid.
Over the years, medical journals have linked nickel allergies to jewelry or coins touching skin, yet the story shifts once nickel lands on the dinner plate. The U.S. FDA and European Food Safety Authority both flagged nickel as potentially allergenic and toxic at higher doses. Long-term studies in rats and mice delivered evidence of organ damage, reproductive issues, and increased cancer risk with hefty nickel intake. Humans see a lower risk threshold, but even a few milligrams per day, especially among sensitive groups, pushes the edge.
Pairing nickel with citrate doesn’t magically erase toxicity. If anything, citric acid can help nickel hop across gut walls, making it more available to the body. This sounds efficient, but it raises red flags for people who react strongly to nickel. Medical case reports describe rashes, stomach pain, and even systemic reactions after consuming nickel-heavy foods or supplements. By making absorption easier, nickel citrate ramps up risk for anyone with nickel sensitivity.
Nickel citrate occasionally lands in dietary supplements, sometimes unlisted. Some companies chase trace mineral blends or try to improve product stability. But the food world rarely includes nickel on purpose, and with reason. Its role as an allergen isn’t just a label issue; it sits at the core of real health threats for a sizeable slice of the population. For anyone with a nickel allergy, accidental consumption can mean weeks of hives, eczema, or worse.
Working in nutrition clinics, I met clients who couldn’t figure out rashes triggered by their “healthy” diets. A handful eliminated nuts, chocolate, or lentils—foods naturally high in nickel. For most, symptoms eased up. That firsthand experience stacks up with the science. Nickel—citrate or otherwise—can mess with immune systems, especially for women, who studies show suffer more from dietary nickel reactions.
Gaps in supplement regulation have left room for accidental overexposure. Reform could include strict labeling whenever nickel compounds show up. Food manufacturers ought to flag nickel in ingredient lists—much like major allergens today. Doctors and nutritionists stay vigilant about warning patients with known sensitivities, but without clear information on packaging, struggles continue.
Anyone with nickel allergies probably won’t gamble on supplements or food containing nickel citrate. Those without allergies still benefit from sticking to balanced diets and keeping an eye on regulatory changes. It always helps to double-check ingredient lists, seek professional advice, and report any strange reactions. While tiny amounts of nickel will always find a way onto our plates, nickel citrate deserves real scrutiny—and better public awareness—when it comes to human consumption.
Supplements catch attention these days, and Nickel Citrate lands in this category. For some, trace minerals carry an appeal. The idea: the body needs a certain level of nickel, just like it does with iron or zinc. Some food—like chocolate, soybeans, and nuts—already packs in a bit. Still, too much of anything pushes the body out of balance, and Nickel Citrate raises some concerns, especially because nickel allergies are pretty common. According to the American Contact Dermatitis Society, nickel ranks as one of the top substances behind skin allergies in the US.
Those sensitive to nickel tend to experience rashes when their skin touches nickel jewelry. Swallowing a nickel supplement like Nickel Citrate can bring out the same itchy red rash, except it shows up anywhere on the body, not just under a ring or on the wrist. This type of reaction is called “systemic contact dermatitis.” Beyond the rash, some people also report hives, and in rare cases, breathing trouble. The body’s immune system sets off on high alert, and things get itchy or swollen fast.
Nickel can also mess with the gut. Nausea, cramps, and diarrhea sometimes follow after a dose, especially on an empty stomach. Studies out of Europe show that high nickel diets can make sensitive folks miserable—headaches, upset stomach, even fatigue—sometimes after just a few days. It doesn’t help that nickel builds up if taken every day. Scientists agree that people with kidney problems face the most risk, since the kidneys filter out nickel. That build-up leads to toxicity, though this happens rarely.
Anyone known to react badly to piercings or cheap earrings shouldn’t take Nickel Citrate without a clear reason. Allergy shots won’t cover this. Once eaten, nickel can worsen eczema or chronic hand dermatitis, a tough skin problem mostly seen in people who wash dishes for a living or work with chemicals. Some people try to solve mystery skin rashes for months before spotting the link to an over-the-counter supplement or even trace amounts in multivitamins. It feels frustrating trying out cream after cream, only to realize that nickel, of all things, might be at the root.
The World Health Organization keeps tabs on nickel in the workplace and notes that long-term exposure—especially for workers in metal factories—leads to higher cancer risk, mostly with inhaled dust. This doesn’t apply to Nickel Citrate pills, of course, but the bottom line is clear: small amounts are safe for most, but it’s easy to go overboard.
One practical step: read supplement labels closely. The U.S. Food and Drug Administration doesn’t tightly regulate trace mineral supplements, so dosing can be off from pill to pill. Doctors and pharmacists stick to the view that nickel isn’t needed for most people, since regular food covers what we use. Anyone with an unexplained rash or sudden digestive trouble after a new supplement should mention every pill, not just the obvious prescriptions. For those with a history of allergies or skin problems, it makes sense to skip Nickel Citrate unless a physician specifically advises it and keeps a close watch.
Science learns more about nickel’s role every year, but basic steps—knowing allergies, watching for side effects, and checking with a healthcare provider before starting—allow people to steer clear of the worst outcomes.
Nickel citrate doesn’t show up in the average cabinet of vitamins and minerals. When I look for research on it, the medical journals don’t flood me with large studies or everyday uses. Nickel itself is a trace mineral. Our bodies actually need it, but in such tiny amounts, you’d miss it if you blinked. Walking down the supplement aisle, you find more talk about magnesium or iron, rarely anything about nickel. That tells me most people just get enough from their regular meals.
The U.S. National Institutes of Health have set minimal guidelines for daily nickel intake, not for supplements but as an upper safe limit for natural intake. For adults, that limit sits at roughly 1 milligram daily, mostly through grains, nuts, chocolate, and even tap water. Healthcare providers don’t actually recommend supplementing nickel unless there’s a specific medical need, and that usually comes after some careful consideration of risks and benefits.
Nickel citrate appeals to researchers working in nutrition science, because nickel helps enzymes carry out reactions in our bodies. At the same time, nickel shows up in allergy studies. I remember reading how some people develop rashes or even more serious reactions after handling coins, wearing jewelry, or coming in contact with nickel-rich foods. Doctors warn those folks against adding nickel to their intake. Getting nickel through a supplement, especially without medical supervision, stays off the table for most.
No organizations offer an official, evidence-based “recommended dosage” for nickel citrate in the way we see for calcium or vitamin C. Reliable government resources focus on safety, not on helping people supplement their diets. If someone’s in a lab setting or working on a clinical trial, doctors might set a dosage, but that doesn’t translate into a safe home-use guideline.
The real trouble starts if people take too much nickel, because even though the body needs a little, too much can build up and become toxic. Short-term symptoms include nausea and stomach pain. Over time, high exposure adds up to bigger risks, including problems with kidney or lung function. If a supplement’s even a consideration, a board-certified doctor or registered dietitian would review the full picture: other health conditions, current medications, diet, allergies, and even work or home exposures.
People who think about supplementing nickel should really ask, “Why do I need this?” If a medical professional finds a true deficiency, they’ll work out how to restore balance, often without a supplement unless diet can’t do the job. Even experienced clinicians rarely see cases needing nickel supplementation.
The supplement industry doesn’t get much oversight. No FDA process reviews nickel citrate for safety or purity in over-the-counter products. That increases the chance of contamination or misleading labels. Trustworthy advice comes from health professionals, and if there’s any uncertainty, blood or urine tests can provide facts, not guesses, about how much nickel someone carries in their body.
Focusing on a varied diet full of grains, seeds, and vegetables gives most people the nickel their bodies require. If someone thinks they need supplementation, or has symptoms after eating certain foods or wearing jewelry, prompt consultation with a physician makes all the difference. No over-the-counter regimen should start without a real diagnosis and guidance. With nickel citrate, less is almost always enough. Knowing the facts keeps health in check and risks manageable.
I’ve spent time looking for specific chemical compounds, so I know the frustration that comes with searching for something like nickel citrate. It often isn’t on the shelf at your local store. Only a handful of chemical suppliers deal with specialty chemicals, and they tend to target schools, research labs, or manufacturing. You probably wonder why it’s so hard to buy a material that seems pretty basic. The answer falls between regulation and safety. Nickel compounds can be harmful if handled incorrectly, and suppliers have to confirm buyers are using them responsibly.
Nickel citrate shows up most often in scientific supply catalogs. Sigma-Aldrich, Alfa Aesar, and Thermo Fisher Scientific come to mind because they’re known for clear sourcing and thorough documentation. If you’re part of a lab or an educational institution, you get access more easily. Safety comes up as soon as toxic metals enter the conversation. Nobody wants to end up inhaling dust or spilling the solution. That’s why these suppliers verify a buyer’s credentials and reason for purchase.
To separate the legitimate sources from risky sellers, check if the company requires a business account, or proof of your research. Reputable companies publish their certifications and safety data right on the product page. That way you know what you’re getting, and how to handle it. The extra formality feels like a hassle, but it pays off in traceability. After a college project went wrong with a mislabelled reagent, I learned that paperwork and safety aren’t just for show.
Sometimes people hope to hop online and buy chemicals from e-commerce giants. It’s tempting, but chemicals like nickel citrate rarely appear for good reason. Strict regulations block their listing on general shopping sites, pushing customers toward specialty suppliers. If an unknown vendor lists nickel citrate with no documentation or clear details, that’s an uphill climb for trust. Lab chemicals don’t belong in the same marketplace as kitchenware. Without proper vetting, there’s a real risk of contamination and fake product.
Many countries list nickel salts as hazardous. In the United States, the Environmental Protection Agency and the Occupational Safety and Health Administration both cover workplace exposure. European nations apply REACH regulations. Suppliers take these rules seriously, so private citizens or hobbyists will hit dead ends. Even for researchers, import and transport can involve maze-like paperwork. Years ago, I ordered a zinc compound for a class, only to get a call from campus safety. They reminded me that importing any metallic salt brings red tape.
If you need nickel citrate for a legitimate purpose—maybe a chemistry project or product development—build relationships with certified vendors. Start early and be upfront about your project and affiliations. If you’re outside a formal lab, talk to local universities or environmental services. Sometimes, joining a makerspace or partnering with a school covers regulatory demand. For anyone just curious, focus on learning and safe substitutes. Ultimately, following the proper channels heads off problems before they get serious.
| Names | |
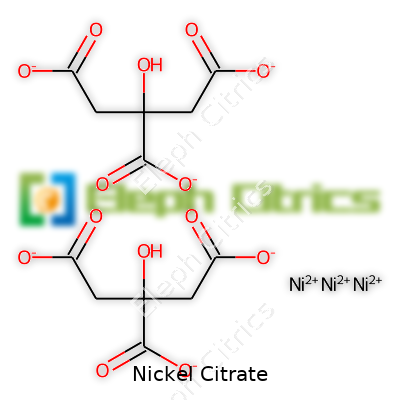
| Preferred IUPAC name | nickel(2+) 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Citric acid, nickel(2+) salt Nickel(II) citrate Citrate de nickel Nickel citrate tribasic |
| Pronunciation | /ˈnɪk.əl ˈsɪ.trət/ |
| Identifiers | |
| CAS Number | 866-81-1 |
| 3D model (JSmol) | `'Nickel Citrate': "C(C(=O)O)C(CC(=O)O)(C(=O)O)O.[Ni+2]"` |
| Beilstein Reference | 3851427 |
| ChEBI | CHEBI:131777 |
| ChEMBL | CHEMBL613028 |
| ChemSpider | 20170348 |
| DrugBank | DB14541 |
| ECHA InfoCard | W-024662 |
| EC Number | 232-104-9 |
| Gmelin Reference | 84880 |
| KEGG | C19612 |
| MeSH | D017366 |
| PubChem CID | 159869 |
| RTECS number | WV8220000 |
| UNII | 7SV29R1GQP |
| UN number | UN3077 |
| Properties | |
| Chemical formula | Ni3(C6H5O7)2 |
| Molar mass | 340.90 g/mol |
| Appearance | White to off-white powder |
| Odor | Odorless |
| Density | 1.88 g/cm3 |
| Solubility in water | Slightly soluble |
| log P | -1.64 |
| Acidity (pKa) | pKa1 = 3.13, pKa2 = 4.76, pKa3 = 6.40 |
| Basicity (pKb) | 7.5 |
| Magnetic susceptibility (χ) | +1700e-6 cm³/mol |
| Dipole moment | 1.74 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 231 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V07AY30 |
| Hazards | |
| Main hazards | May cause allergy or asthma symptoms or breathing difficulties if inhaled. May cause an allergic skin reaction. Suspected of causing cancer. Toxic to aquatic life with long lasting effects. |
| GHS labelling | Warning; H317, H319, H334, H335, H341, H351, H372, H412 |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | H317: May cause an allergic skin reaction. |
| Precautionary statements | P261, P280, P302+P352, P304+P340, P308+P313, P314, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 2-0-0 |
| Lethal dose or concentration | LD50 oral rat 320 mg/kg |
| LD50 (median dose) | LD50 (median dose) Oral rat > 5000 mg/kg |
| NIOSH | WA2275000 |
| PEL (Permissible) | 1 mg/m³ |
| REL (Recommended) | 0.3 mg |
| Related compounds | |
| Related compounds |
Iron(III) citrate Cobalt(II) citrate Copper(II) citrate Nickel(II) sulfate Nickel(II) acetate |