Potassium bismuth citrate has a story that comes with deep roots in the development of pharmaceuticals. Its formation traces back over a century as researchers sought gentle treatments for stomach ailments. Bismuth-based compounds carried forward a reputation for soothing gastric discomfort even before the structure of this particular citrate was fully described. Early chemists noticed that combining bismuth salts with organic acids enhanced solubility and patient tolerance. Over time, this compound took shape based on trial, observation, and cautious experimentation. Hospitals and clinics relied on bismuth drugs not just for relief, but for protection of the stomach lining at a time when few alternatives existed. This context helped potassium bismuth citrate shift from a curious laboratory salt toward a product embedded in the practice of medicine.
Potassium bismuth citrate stands out as a complex salt combining bismuth, potassium, and citric acid. Its shiny, white appearance and mild tang hint at both its metallic and organic origins. In tablet or suspension form, it serves as a primary ingredient in some gastrointestinal medicines. From powders sold to industry to over-the-counter combinations, manufacturers supply it worldwide. Product formulations must hit tight purity levels and particle specifications. For most, this citrate type balances solubility in water with a gentle action along digestive tissue. Modern packaging typically shows this ingredient listed in clear terms for consumers and regulators who expect transparency.
Physical properties matter for potassium bismuth citrate, from its highly crystalline white powder to the slightly acidic taste it leaves on the tongue. High purity samples remain stable under dry, cool storage but may clump if exposed to humidity. Chemically, this complex blends bismuth with potassium ions and citrate as a tricarboxylic acid ligand, forming a salt with low bioavailability outside of the intended environment in the gut. Its solubility profile, moderate in water but poor in most organic solvents, aids product design for safe ingestion. The molecule’s integrity holds up under typical manufacturing and shipping stresses, adding confidence for medical use.
Manufacturers of potassium bismuth citrate submit material for strict quality controls. Purity often reaches above 98%, with remaining traces usually restricted to water and minor inorganic salts. Heavy metal residues must drop to near zero, particularly lead and arsenic. Particle size distribution appears on technical datasheets and product analysis certificates. Labels need to reflect not just the precise salt composition, but batch traceability, exact expiry dates, and confirm any additional additives or excipients blended into the product. Standards from USP, EP, or regional pharmacopeias provide the backbone for compliance worldwide, as pharmaceutical producers face government inspection and random spot-checks.
Synthesizing potassium bismuth citrate starts with dissolving bismuth nitrate in a controlled acidic medium, followed by gradual addition of citric acid and potassium carbonate. The reaction releases carbon dioxide, prompting a gentle bubbling action, as pH is measured closely at every step. Careful stirring helps crystals take shape slowly without forming large aggregates. After full reaction, filtration removes insoluble byproducts, and the remaining solution undergoes evaporation or cooling to precipitate pure potassium bismuth citrate. Washing and drying follow, yielding a fine, ready-to-package or further refine powder. Lab teams often tweak conditions for maximum yield and consistency, showing just how much technical precision ties into pharmaceutical salt production.
Potassium bismuth citrate reveals much about its inner structure through its chemistry. Acidic conditions keep the citrate stable, yet strong mineral acids like hydrochloric can displace the bismuth. Alkali addition sometimes triggers breakdown, releasing bismuth salts or citrates with simpler geometry. Heat speeds decomposition, splitting it into potassium salts, bismuth oxide, and carbon dioxide. Lab chemists sometimes modify the parent structure by introducing other biocompatible metals, exploring if hybrid salts offer new medical benefits. Subtle tweaks to the citrate:metal ratio lead to alternate solubility properties, which encourages research into specialized delivery systems for targeted therapy.
Across the globe, this compound pops up under several names. “Potassium bismuthyl citrate,” “tripotassium dicitratobismuthate,” and “bismuth potassium citrate” come up in chemical catalogs. In the marketplace, a familiar trade name is De-Nol, commonly found in Asia and parts of Europe. International scientists stick with chemical nomenclature for grant proposals and research papers, but pharmacists and physicians typically shorthand the ingredient. This range of synonyms sometimes creates confusion for newcomers, and good practice calls for checking batch codes and chemical identities every time a purchase is made.
Handling potassium bismuth citrate in manufacturing settings calls for strict protocols. Direct skin contact rarely triggers problems, but prolonged exposure can cause irritation. Inhalation of powder dust must be avoided, not just to protect the lungs, but to prevent chronic uptake of minerals. Facilities train line workers with standard PPE routines—gloves, dust masks, and eye protection stand as basics. Storage rooms keep stocks dry, sealed, and protected from heat sources. Waste management collects any spilled or out-of-spec powder for controlled disposal. Most importantly, clear documentation across all steps underpins compliance, as pharmaceutical salt handlers work under frequent audits and strict batch-control regulations.
Pharmaceutical use leads the way for potassium bismuth citrate. As a mainstay in anti-ulcer therapy, it targets Helicobacter pylori and soothes inflamed gastric mucosa. General practitioners, especially in Asia, often recommend courses containing this ingredient for dyspepsia and occasional digestive distress. Its gentle coating action shields stomach lining from acid assault, helping people push back pain without the harsher effects of proton pump inhibitors. Scientists and hospital pharmacists measure its impact across hundreds of thousands of patients who need symptom relief without disrupting systemic metal balance. Veterinary applications sometimes draw on its GI tract protection as well, though strict regulations keep medical-grade supplies separated by species.
Pharmaceutical labs continue to seek out new frontiers for potassium bismuth citrate. University teams investigate tweaks to structure, hoping to fine-tune selectivity against bacteria. Mass spectrometry and other analytical tools help determine how minor impurities influence both efficacy and safety. Nutritional researchers draw on its unique structure to probe mineral transport in the human body, with results guiding further refinement of drug design. Grant funding often follows global health needs, especially given the growing resistance among gut pathogens. Industry-led research, on the other hand, keeps its eyes on lowering production costs and increasing storage life while maintaining potency. Every year sees a steady stream of new literature exploring ways this molecule could drive safer and more effective therapies.
Decades of safety testing back the medical use of potassium bismuth citrate, but that hasn’t stopped toxicologists from asking pointed questions. Early studies flagged signs of nervous system impact only with chronic, high-dose exposure. Modern regulatory authorities set strict daily intake limits and demand batch testing for side compounds. Teams examine not just acute reactions, but how long-term, low-dose use interacts with patient comorbidities. Case reports get tracked worldwide, ensuring early warning if patterns shift. Animal testing continues for new derivatives, and sophisticated cell models catch even subtle signs of toxicity missed in the past. All this tight oversight underscores both commitment to patient wellbeing and the strong record of safety in approved settings.
Looking ahead, the role of potassium bismuth citrate may shift as more is learned about stomach health and the human microbiome. With resistant bacteria spreading and the world demanding new stomach-protecting drugs, companies stay keen on tweaking the molecule for smarter delivery, stronger antibacterial activity, and fewer side effects. Partnerships between universities and drug firms encourage new clinical applications, not just as stand-alone agents but as part of combination therapies. Researchers in materials science eye this compound’s structure for inspiration in green chemistry and as a model for other bismuth-based drugs. With a rich track record behind and ongoing demand, potassium bismuth citrate seems set to retain a place in both laboratory and clinical medicine for years to come.
Potassium Bismuth Citrate lands on a lot of medicine shelves as a key ingredient in over-the-counter stomach relief products. I remember my grandmother always having a bottle in her cabinet for those long nights when heartburn felt like it could knock you out. This compound tackles symptoms of indigestion, heartburn, and upset stomach by protecting the lining of the gut. It coats the stomach walls, helping to reduce irritation and calm the beast many call reflux. Unlike some chemical fixes that just mask symptoms, this compound pulls its weight by helping to reduce inflammation and blocking irritating agents. Clinical studies report that bismuth compounds can actually help manage certain peptic ulcer disease and mild gastrointestinal infections. This practical approach means fewer sleepless nights bent over the sink, looking for relief.
In recent years, doctors have pulled Potassium Bismuth Citrate into the spotlight for another reason: fighting infections tied to ulcers. A stomach bug called Helicobacter pylori has a knack for burrowing into the stomach lining, setting up shop for chronic pain and even risking ulcers or cancer if left alone. Combination therapies, often mixing this compound with antibiotics and drugs like proton pump inhibitors, help knock out stubborn infections. The bismuth targets bacteria directly, while offering a layer of protection, giving antibiotics a better shot at clearing the problem. It’s not an old wives’ tale—research backs up this triple therapy approach, showing higher cure rates and better protection for the stomach than antibiotics alone.
Beyond home remedies, this compound plays a clever role in medical labs. Some diagnostic procedures rely on Potassium Bismuth Citrate to prepare tissue samples or create controls for specific chemical reactions. Bismuth's chemistry makes it a smart choice when a stable, non-reactive ion is needed, especially compared to more aggressive metals like lead. In my years talking with hospital chemists, more than one has pointed out how these salts make life easier by avoiding toxic fumes and offering reliable results.
Pharmaceutical companies rely on Potassium Bismuth Citrate thanks to its predictable stability and solid shelf life. Unlike some metal compounds that break down fast or create nasty byproducts, this one holds up well in long-term storage and isn’t fickle about heat. Manufacturers use it to standardize dosages, so patients get what they need, not an under- or overshot mess. My own stint working at a small pharmacy showed me the difference—mixing powders and solutions with bismuth compouunds had fewer headaches when it came to quality checks.
No chemical tool comes free of concerns. Potassium Bismuth Citrate, if overused, can cause its own problems—like dark stools or, rarely, more serious side effects in folks with kidney trouble. Good communication between patients and doctors remains a must. Researchers continue hunting for ways to make bismuth-containing drugs safer, even exploring newer delivery methods to dodge side effects. For those in the industry, updating safety guidelines and investing in clear patient education should never fall off the radar.
Potassium bismuth citrate turns up in medicines targeting stomach discomfort. It often brings relief to people with indigestion, gastric ulcers, and even some mild infections caused by bacteria like Helicobacter pylori. The ingredient appears in many over-the-counter antacids and is sometimes preferred because of its metal-based profile, which can act as a barrier over irritated stomach linings. But when people ask about long-term safety, concerns about how much the body can handle over time come to mind.
Doctors and pharmacists keep a close watch on patients taking metal compounds for extended periods. Medical journals have covered bismuth toxicity since the late 1970s. Most problems show up only after months of daily use and usually at doses above those found in common indigestion pills. Side effects can range from darkening of the tongue and gums to rarer neurological problems, including confusion and unsteady movement. Several studies have tracked patients taking potassium bismuth citrate for weeks to months under prescription guidance and found little risk at standard doses—though they all suggest people avoid exceeding recommended amounts without medical supervision.
I remember my grandmother keeping bismuth-based tablets next to her teacups. She took them after spicy meals and swore by their stomach-soothing qualities. She never faced any major health scares, but her doctor kept a careful eye on her medications since she took them often. This kind of oversight matters, especially for older adults, folks with kidney problems, or people using several drugs at once. Bismuth does not stay in the body for long in healthy people, but in those with kidney issues, it can build up. That’s when side effects pop up more often.
Doctors across the world report that most side effects connected to potassium bismuth citrate fade quickly after stopping the drug. People often notice harmless dark stool or mild changes in taste, which resolve within days. The more serious issues tend to show up after months of very high or unmonitored dosing. Even so, scientists and regulators have set strict guidelines for maximum safe dosages. They base those on rigorous clinical studies, reviews, and safety monitoring. Well-known regulatory agencies, including the U.S. FDA and the European Medicines Agency, support its short-term use and monitor ongoing research for any changing evidence.
Medicines that people use over long periods need careful review every year. Patients should check with their doctor before using potassium bismuth citrate as a daily supplement, even if they buy it over the counter. Pharmacists help, too, by tracking drug interactions and advising on safe dosing. Companies that make these products need to keep packaging clear and easy to understand, so people can avoid mistakes. As with most medicines, the right dose, regular monitoring, and honest communication between patient and doctor go a long way in making long-term use safer.
Questions remain about rare side effects and how this compound interacts with other chronic medications. Researchers continue tracking those who use it for conditions beyond simple indigestion. Anyone worried about ongoing symptoms should ask their healthcare provider if another treatment fits their health story better. As research pushes forward, scientists might find new guidelines or improvements, but so far, keeping an eye on dose and duration stands out as the best precaution.
Potassium Bismuth Citrate plays a role in medicine, especially for stomach problems. Doctors use it to fight Helicobacter pylori, the bacteria behind many ulcers. People reach for it to manage heartburn or indigestion. This compound isn’t new—but most folks don’t know much about its side effects.
Some take Potassium Bismuth Citrate and start feeling queasy. Nausea pops up for certain people, sometimes leading to vomiting. The taste in the mouth often feels metallic or downright weird. Stomach pain or cramps might turn a simple treatment into an uncomfortable chore. I’ve spoken to patients who swear their appetite drops off after a few days on this medication. Constipation happens, too. That’s uncomfortable, especially for older adults. A change in stool color can catch people by surprise. Black stools don’t always mean bleeding—this medicine turns things dark as a byproduct, much like other bismuth compounds.
While rare, some users report headaches or dizziness. A small percentage talk about ringing in the ears—tinnitus—they never expected from a stomach remedy. These side effects usually fade when people stop the medication. Still, anyone who feels unsteady or confused should stop and talk to a doctor straight away. From experience, these issues seem more likely in folks taking big doses or combining bismuth with other medicine that affects the brain.
A strange side effect nobody mentions enough: bismuth can turn the tongue or gums black. This looks alarming but most times it’s harmless and brushes off after treatment stops. Sometimes people notice mood changes or trouble sleeping, though this appears less common and studies haven’t nailed down a direct link. Allergic reactions like rashes, itching, or hives can develop, especially for those sensitive to bismuth or citrus-based compounds. Severe allergies carry real risk—swelling in the face or difficulty breathing needs immediate medical attention.
The kidneys work hard to clear out metals and minerals. Long-term or high-dose use of Potassium Bismuth Citrate can stress these organs. Over time, bismuth may build up in body tissues. Medical literature documents cases where people using bismuth compounds long-term developed problems like confusion, muscle weakness, or even tremors. These aren’t common, but they do make careful use essential. I remind anyone with kidney problems—or those taking other heavy-metal medicines—to keep their doctor in the loop.
Doctors usually prescribe the lowest dose for the shortest time. Sticking to this plan lowers risk of side effects. Reading the label, tracking any strange changes in mood or health, and asking questions at appointments helps people use this treatment wisely. People who notice severe reactions need to stop the medicine and get checked out. Sharing your full medical history with the doctor makes a real difference. In my time working with patients, the best outcomes come when people stay open about symptoms and doctors pay close attention to their feedback.
Matching the right drug to each person matters more than ever. If someone reacts to Potassium Bismuth Citrate, alternatives exist—though they come with their own side effects and strengths. Regular reviews with a trusted doctor or pharmacist help catch trouble before it starts. In some cases, diet changes or different medicines clear up stomach issues without the need for bismuth. For anyone worried about side effects, clear communication and shared decision-making pave the way to better health.
Potassium Bismuth Citrate shows up in labs, pharmacies, and research centers more often than most realize. My time in the lab taught me simple mistakes with storage can mean big headaches later—wrong color, clumping, or something worse like degradation that ruins test results or stability. Reliable work and safety both start with smart care after the shipment gets signed in. Let’s talk real-life ways to keep this compound stable and safe for everyone in the building.
Moisture and this powder don’t mix. Even a little humidity from air sneaking into a jar can trigger changes. I’ve seen samples clump in a day, reducing their usefulness. Contamination risks creep up, and the powder might even lose some of its effectiveness. Air-tight containers make a serious difference. Lab managers swear by high quality, screw-top jars with rubber gaskets, storing them right after each use. That’s not overkill; mold, unexpected reactions, and wasted inventory all cost way more than decent storage gear.
Temperatures above 25°C can start to mess with the chemistry of Potassium Bismuth Citrate. But sticking it in just any fridge isn’t wise—cycles of cooling and warming fight against stability, especially if people open the fridge every ten minutes. Dedicated cool cabinets, temperature logs, and keeping products on shelves instead of the floor works. Light-sensitive reactions creep in if the powder sits on a shelf near big windows or overhead bulbs. Amber jars or keeping containers in a closed, dark cupboard provides a simple fix. Some facilities invest in UV-block cabinets, but the biggest difference comes from not leaving it out at all.
Even top researchers lose track of expiration dates. Clear labeling with bold writing cuts down on mix-ups. I learned to write open dates and batch numbers right where they can’t rub off. That’s helped my colleagues avoid dosing errors or invalid lab results more than once. Out-of-date product doesn’t just disappoint; it has a real chance of giving the wrong answer in sensitive tests, which can ripple through clinical studies or patient results.
Some folks still reach scoops into jars or double-dip spatulas. Cross-contamination ruins purity fast. Separate, clean scoops every time make life simpler. Companies invested in single-use tools and strict clean-up routines, and their incident rates dropped right along with it. Habit makes or breaks any process, so real training—not just posters on a wall—turns good storage into an everyday habit.
Expired or degraded Potassium Bismuth Citrate shouldn’t end up in the regular trash. Proper hazardous waste channels keep labs legal and prevent environmental headaches. I’ve watched what happens when protocols get lazy: surprise audits, fines, and extra paperwork nobody wants. Label waste clearly and keep outdated product together for scheduled pick-up by licensed disposal teams.
The science behind Potassium Bismuth Citrate stays strong when people treat it right. Storing it dry, cool, and in the dark, labeling well, and avoiding contamination or mishandling keeps both results and people safe. Simple habits, reinforced with steady training, do more than any fancy equipment or new tech. In my years under the fluorescent lights, nothing has replaced respect for the basics.
People often reach for remedies like Potassium Bismuth Citrate to ease stomach issues. In my years helping patients manage digestive complaints, I’ve seen folks juggle a handful of pills picked up over the counter or prescribed by the doctor. Someone dealing with heartburn or an upset stomach may have a medicine cabinet full of options—and not much guidance on which ones play well together.
Doctors suggest Potassium Bismuth Citrate for mild stomach upsets, indigestion, or some cases of diarrhea. Bismuth salts have a long history in medicine. They can help knock out mild infections in the gut and settle the stomach lining. Unlike some harsher meds, people report fewer side effects with bismuth than with the antibiotics sometimes used for severe stomach infections.
But, even something that seems gentle can cause trouble when mixed with certain other medications. Some antacids or acid reducers will either block or boost the effects of Potassium Bismuth Citrate. This means someone looking for quick relief might accidentally lessen the power of both drugs or raise their risk of side effects without realizing it.
Mixing medicines isn’t just a pharmacist’s puzzle—it has real human costs. The most common problem I see is someone pairing Potassium Bismuth Citrate with old-school antibiotics like tetracycline. These can bind together in the gut, making the antibiotic too weak to do its job. If someone is using both for a stomach infection, that combination could drag out an illness much longer than needed.
Then there’s the question of kidneys. People who take blood pressure drugs—especially ACE inhibitors or diuretics—already keep a close eye on their lab values. Adding more potassium from Potassium Bismuth Citrate can sneak potassium levels up, tipping the balance and putting extra strain on the kidneys. Anyone dealing with chronic kidney disease should tread carefully, since excess potassium can have dangerous effects on the heart’s rhythm.
Sorting out which meds work together isn’t always straightforward. Doctors and pharmacists use trusted databases and drug reference tools; not everyone has access to those in their daily lives. In practice, a short talk with a healthcare provider saves endless guessing. A lot of people keep a running list of what they take and share it each time they get a new prescription or over-the-counter medication. That habit alone flags problems early, like risky combinations with potassium or bismuth salts.
The packaging on Potassium Bismuth Citrate often carries a warning about taking it close to other medicines. Good advice usually means leaving a gap of a few hours before or after swallowing other pills. That breaks up the chance for the drugs to tangle in the stomach or block each other’s effects.
What’s really missing is clearer public messaging. Too many people trust old advice, picking up remedies based on family or internet tips. With drug interactions, even a well-meaning mistake can slow down healing or pile on new side effects. Consumer leaflets, pharmacy consultations, and trustworthy medical websites all help fill the gap, but busy lives mean people miss out on updates. Giving folks simple charts or checklists would help sort out which meds don’t mix and flag when to ask a professional.
No one wants to spend extra time battling an upset stomach—or a dragged out antibiotic course. Being honest at the doctor’s office and keeping meds in plain sight at home helps avoid these silent risks. In the end, clear communication and practical advice matter most. Health stays manageable when each medicine earns its spot in the daily routine, not when medicines turn into each other's stumbling blocks.
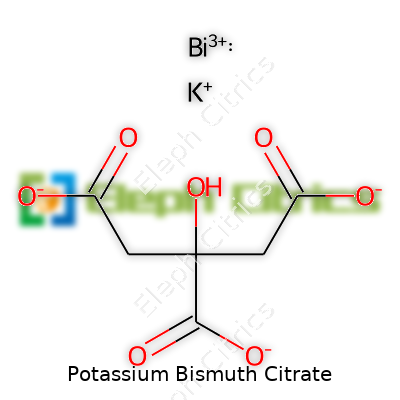
| Names | |
| Preferred IUPAC name | Tripotassium trisbismuth(3+) hexakis(2-hydroxypropane-1,2,3-tricarboxylato(3−)) |
| Other names |
Bismuth Potassium Citrate Tripotassium dicitratobismuthate Potassium subcitrate bismuth |
| Pronunciation | /pəˈtæsiəm ˈbɪzməθ ˈsɪtrət/ |
| Identifiers | |
| CAS Number | CAS Number: "20665-61-0 |
| Beilstein Reference | 358268 |
| ChEBI | CHEBI:53497 |
| ChEMBL | CHEMBL2108506 |
| ChemSpider | 22211302 |
| DrugBank | DB09294 |
| ECHA InfoCard | echa.europa.eu/substance-information/-/substanceinfo/100.108.244 |
| EC Number | 242-232-6 |
| Gmelin Reference | 114714 |
| KEGG | C18610 |
| MeSH | D019371 |
| PubChem CID | 166829 |
| RTECS number | GG5960000 |
| UNII | UF19U183RQ |
| UN number | UN2811 |
| Properties | |
| Chemical formula | K₆Bi₄(C₆H₃O₇)₄ |
| Molar mass | 832.52 g/mol |
| Appearance | White or almost white crystalline powder |
| Odor | Odorless |
| Density | Density: 2.44 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -0.4 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 1.2 |
| Basicity (pKb) | 12.8 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.65 |
| Dipole moment | 1.82 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 512.8 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | A02BX05 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | Keep container tightly closed. Store in a dry place. Store in a well-ventilated place. Wear protective gloves/protective clothing/eye protection/face protection. Wash hands thoroughly after handling. IF ON SKIN: Wash with plenty of water. |
| NFPA 704 (fire diamond) | 1-1-0 |
| Lethal dose or concentration | LD50 (oral, rat): > 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) of Potassium Bismuth Citrate: "2,000 mg/kg (oral, rat) |
| NIOSH | Not Listed |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.3~0.6g |
| Related compounds | |
| Related compounds |
Bismuth Subcitrate Bismuth Subsalicylate Potassium Citrate Bismuth(III) Oxide Bismuth Citrate |