Potassium citrate monohydrate has deep roots that stretch back hundreds of years, showing up in old medicines as early as the eighteenth century. An early form of potassium citrate started as a treatment for scurvy and other conditions where people wanted to restore or balance body fluids. Chemists learned to combine citric acid with potassium sources, giving doctors a way to produce a gentle, slightly salty compound for patients. Over the years, extraction and purification stepped forward. By the twentieth century, manufacturing grew more precise and scalable. Instead of relying on plant extracts, industry shifted to controlled synthesis, improving purity, making granules and powders with reliable potency—something you can’t get from old herbal tinctures. Health and nutrition sciences have continued to explore potassium citrate, moving it beyond apothecaries into a chemical used across food, pharmaceuticals, even industrial and lab-scale procedures.
You’ll find potassium citrate monohydrate as a white to colorless, crystalline powder. It comes mildly alkaline, easily dissolved in water, and tasteless enough for easy blending with syrups, pills, tablets, or food mixes. Manufacturers sell it under several trade names, making it simple to locate in ingredient lists on processed food, dietary supplements, or medical supplies. Medical use keeps growing, as potassium citrate offers a gentle approach for managing urinary stones, balancing electrolytes in kidney care, and protecting against certain forms of metabolic acidosis. In personal experience, consulting for a food company revealed just how valuable potassium citrate’s buffering action can be for stabilizing acidity and improving shelf life, especially in canned and processed foods.
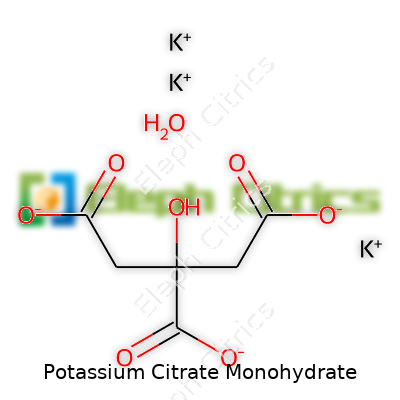
A closer look under the microscope shows potassium citrate monohydrate’s formula: C6H5K3O7·H2O. Crystal particles reflect light in a glassy, almost transparent way. Its density sits around 1.98 grams per cubic centimeter and it melts slowly above 180°C, gradually losing water. Water takes potassium citrate quickly—stir a spoonful into a glass and it vanishes, making it easy to use wherever pure potassium is needed without the sharp tang of other mineral salts. Its solubility reaches about 33 grams in 100 milliliters at 20°C, so even low temperatures won’t prevent effective mixing.
On technical sheets, potassium citrate monohydrate carries strict purity requirements, usually higher than 99%. Grades differ based on end-use: food-grade must meet standards for heavy metals—like lead and arsenic—while pharma-grade goes through tighter controls, including absence of microbial contaminants and residual solvents. Labeling reflects these criteria—ingredient lists mark the additive as either “Potassium Citrate Monohydrate” or its E-number (E332), depending on local regulatory forces. Storage advice often recommends a dry, sealed environment, since the monohydrate absorbs moisture and loses weight if left open to the air. Barcodes and batch data keep traceability strong, giving authorities and buyers control over safety and consistency in the event of recalls or disputes.
The standard method starts with citric acid, extracted from citrus fruits or produced by fermentation with Aspergillus niger. Potassium carbonate or potassium bicarbonate gets added, triggering a neutralization reaction. The mixture forms potassium citrate and releases water and carbon dioxide. Careful control of temperature and ratios is crucial: too little potassium, and conversion drops; too much, and unwanted byproducts creep in. Once fully reacted, the liquid gets concentrated through evaporation, letting potassium citrate crystals settle out. These crystals get dried and ground, then blended with extra water for monohydrate stabilization. Modern systems focus on closed processes to avoid contamination. My own attempts at small-scale crystallization for educational purposes underscore the importance of thorough drying to keep a stable, free-flowing powder that is ready for precise weighing in laboratory and industrial routines.
Potassium citrate acts as more than just a basic salt. It can link up with acids or bases, and—thanks to its tricarboxylate structure—form stable complexes with metals (like magnesium and calcium) or break down in heat to give potassium carbonate. Its gentle, buffering effect softens pH swings, a major asset for food applications. In reactions, potassium citrate’s chelating properties find use in cleaning, water treatment, and lab analysis, often providing stronger binding than simpler potassium salts. Chemists have developed various forms, including anhydrous versions and blends with other mineral supplements, offering flexible approaches to specific medical or technical challenges.
Check ingredient labels and you might spot potassium citrate monohydrate listed as tripotassium citrate monohydrate, E332, or 2-hydroxy-1,2,3-propanetricarboxylic acid, tripotassium salt, monohydrate. These long scientific labels can sound intimidating, but they point to the same structure. Companies may market potassium citrate under brand names—examples include Kalinex, Citro-K, Urocit-K—each spelling out a particular strength or usage for the pharmacy or consumer market. In food and drink, the E-number system helps authorities and shoppers flag additives quickly, which is vital given media attention and public worries about “hidden chemicals” in what we eat or drink.
Safety starts with handling: potassium citrate monohydrate is mostly benign, but exposure in powder form can irritate the lungs or eyes. Protective gloves, dust masks, and eye shields form part of every workplace’s standard operation. In pharmaceuticals and food manufacturing, batch documentation tracks every step from raw material to final lot, keeping cross-contamination in check. Safety certifications—like ISO 22000 for food safety and GMP for pharmaceuticals—cover potassium citrate’s journey. Disposal needs careful control; excess potassium alters soil and water mineral balances, sometimes harming sensitive ecosystems. Emergency protocols for accidental spills limit environmental exposure and keep workers protected. These real-world practices often go overlooked when folks buy an over-the-counter supplement, but they keep products high quality and free of contamination—and should remind consumers about the sheer scale of testing baked into every simple bottle of potassium citrate.
Application spans several fields. Medical guidelines recommend potassium citrate as a front-line option for kidney stone prevention—raising the pH of urine and increasing citrate concentration reduces calcium stone re-formation. Doctors prescribe it for some kinds of metabolic acidosis, especially in kidney disease, where loss of acid–base balance can grow dangerous. Food technologists rely on potassium citrate to buffer acidity in jams, jellies, soft drinks, and processed cheese, offering a clean-label way to ensure safe, stable foods. Beverage makers use it to tweak both taste and mineral content, creating so-called “electrolyte drinks” that target athletes or anyone seeing salt loss. In personal experience, working with environment remediation specialists, even water treatment facilities have started using potassium citrate as an environmentally friendly chelator to manage certain heavy metals, keeping drinking supplies pure without turning to harsher chemicals.
Current research looks at both human health and new technical roles. Pharmaceutical studies focus on better delivery systems—slow-release tablets, liquid formulations with improved taste and minimal gastrointestinal discomfort. Food researchers investigate the interaction between potassium citrate and flavor compounds, preserving nutrients during pasteurization and helping manage sodium intake by cutting back on table salt and relying on potassium-based salts for health benefits. Materials researchers study potassium citrate as a growth medium in developing green batteries, or in stabilizing proteins and enzymes for advanced biotech manufacturing. Regulatory shifts also drive R&D, as agencies demand additives that deliver maximum function at lower doses with better scrutiny on long-term effects. Through long partnerships with university research teams, I’ve seen potassium citrate show unexpected new uses in stabilizing plant-based drinks and in enhancing the absorption of mineral supplements for patients with specific dietary restrictions.
Toxicological assessments point toward a safe profile, as long as potassium citrate is consumed within established limits. Overdosing brings risks—the kidneys can only process so much potassium, especially in those with pre-existing renal problems. Side effects at high doses include muscle weakness, gastrointestinal symptoms, and, in rare cases, serious cardiac arrhythmias due to elevated potassium levels in blood. Chronic, low-level exposure shows no serious side effects, but pharmaceutical-grade labels always warn people about using potassium supplements without medical supervision. The FDA and European Food Safety Authority monitor both acute and chronic toxicity data, setting daily intake ceilings. Testing in animals and clinical trials in humans continue, especially as potassium citrate makes its way into a bigger share of supplements on store shelves and into the diet of populations with high rates of kidney disease or heart conditions.
Potassium citrate monohydrate stands to play a bigger role in personal and community health, as societies battle increasing rates of hypertension and cardiovascular disease linked to high sodium consumption. As more consumers and food manufacturers pivot toward potassium-enriched products, potassium citrate’s neutral flavor and strong solubility keep it appealing and easy to include. Regulatory changes may bring new labeling, stricter maximum dose requirements, and tougher reporting for manufacturers, all aiming to protect vulnerable populations and improve public transparency. Research into potassium citrate’s use in battery technology, biodegradable plastics, or medical devices could open up new market niches, turning what was once just a gentle medical additive into a cross-industry mainstay. Those working in R&D, food innovation, or pharmaceutical science should keep potassium citrate on the radar, not just for what it already does but for what further research and problem-solving could allow it to accomplish in the coming years.
Potassium citrate monohydrate comes off as a mouthful, but anyone who’s dealt with kidney stones or has managed a chronic health condition in the family would recognize its value. It’s a simple, white crystalline powder that dissolves easily in water and tastes only mildly salty. In my days working in a hospital pharmacy, I saw how common it was on prescription pads, offering people a practical way to tackle real health challenges.
Doctors often use potassium citrate to help folks manage kidney stones, especially stones formed from uric acid and certain types of calcium. It raises the pH of urine, making conditions less comfortable for stone formation. Patients who stick to the regimen notice that their stone recurrence drops, and the science backs it. Research published in the New England Journal of Medicine reports a clear reduction in stone formation rates among those using potassium citrate.
People battling metabolic acidosis, a trouble that often shows up for those with chronic kidney issues, also find potassium citrate helpful. It balances body chemistry and helps keep bones from losing too much calcium. More than once, I listened to nephrologists explain to worried parents how this supplement supports growth in kids with kidney problems.
Anyone dealing with gout knows the pain that comes with high uric acid. Potassium citrate pushes urine to a more alkaline state, supporting the body’s effort to flush out uric acid. For many, prescription and over-the-counter options with this ingredient make a difference in daily comfort.
The food industry gives potassium citrate a role as well. It acts as an acidity regulator and stabilizer, showing up in drinks, dairy products, and processed foods to keep taste fresh and texture steady. The FDA lists it as generally recognized as safe (GRAS), which gives manufacturers some reassurance. Observing people with low-potassium diets, such as athletes and those with intense training schedules, I’ve seen it added to electrolyte mixes to help prevent dangerous dips in potassium levels.
Pet care also picks up on potassium citrate. Vets prescribe it to cats and dogs with urinary issues, aiming to keep their urine at a friendly pH. This mirrors its use in people and reflects how animal medicine often borrows smart solutions from human health care.
A major challenge is getting enough without risking too much. Potassium in large doses puts hearts at risk, especially for older adults or anyone taking medications like ACE inhibitors. During patient consultations, I always stressed the need to work closely with doctors to keep tabs on blood potassium. Over-the-counter supplements tempt some, but without supervision, the risks start to add up.
Drug shortages create their own headaches. When potassium citrate ran low in the early 2020s, I saw patients scramble for alternatives. It highlights how fragile supply chains can upend lives. Establishing better domestic production and reliable supply sources could soften that blow.
A step forward would be wider education for patients. The science stays solid, but misuse still crops up—either from not taking enough or overlooking other health conditions. Building more partnerships among doctors, pharmacists, and dietitians could help people understand the best ways to use potassium citrate.
Safe disposal of unused product should also get more attention. Environmental guidelines often fail to keep up with the rising use of specialty supplements, so clear instructions help both people and planet.
Potassium citrate monohydrate shows up in my conversations with urologists and people managing kidney stones more than I ever expected. Doctors sometimes write it up to keep certain types of kidney stones from coming back and, less commonly, to handle metabolic acidosis in folks whose bodies have a hard time keeping their acid levels in check. Most people don't start googling this stuff unless a prescription brings it up, and it’s not something folks want to mess around with on their own.
Potassium is one of those minerals the body needs, but doctors can’t just pick a number off a chart for everyone. A lot depends on kidney function, medical history, and blood test results. For kidney stone prevention in adults, typical starting dosages fall around 20 mEq (milliequivalents) two or three times daily, usually after meals. That doesn’t mean every person gets that amount. Some get less, some get more, based on how their bodies handle potassium, any current medications, or other ongoing conditions. Safety matters because potassium builds up fast, and high levels can wind up harming the heart or nerves.
Having seen a family member wrestle with kidney stones, I remember the routine weigh-in at the doctor’s office, conversations about recent blood tests, and reminders not to take extra potassium from supplements or sports drinks. The body’s own filtering system relies on a careful balance, and not everyone’s kidneys can filter potassium equally well. Overdoing it can trigger muscle weakness, irregular heartbeat, or worse. A healthcare professional checks up with blood tests. Over-the-counter labels can't do that job — it takes a real conversation and follow-up with a doctor or pharmacist.
The American Urological Association states that potassium citrate lowers stone formation in patients with low urinary citrate. Randomized clinical trials have found fewer kidney stone recurrences when compared to people not receiving citrate supplements, as long as the dosage suits the patient’s needs. The U.S. National Library of Medicine notes reported adult doses up to about 100 mEq per day, but most people sit somewhere in the 30 to 60 mEq range, spread out through the day. Children take less, based on body weight, and reflecting how much potassium their bodies can safely process.
I remember the relief in my own family after regular appointments kept potassium levels in check. If potassium builds up, doctors sometimes lower the dosage or take a break and repeat blood work. Some people keep track of their foods, watching salt substitutes and processed foods that sneak in extra potassium. Talking to your doctor stays the best approach, especially if you have kidney disease, heart issues, or take water pills (diuretics).
For anyone starting potassium citrate monohydrate, regular blood monitoring and check-ups lower risks and help catch side effects early. Each person has their own safe range, based on labs and real-life experience—not just the numbers on a bottle. Prescribers can adjust the dose over time, aiming for benefits without letting potassium shoot too high. If a pill or powder seems like an easy solution, the real fix comes with a care plan that looks out for the whole person, backed by lab results and honest conversation.
Doctors prescribe potassium citrate monohydrate for people struggling with kidney stones or with low potassium. Modern diets often lack potassium, especially with so many processed foods around. I’ve seen family members get this prescription after a tough bout of kidney pain, and while the relief can be quick, that little white pill brings its own set of baggage.
Experience tells me that no pill works in isolation; most medicines, even useful ones, have a flip side. Potassium citrate can cause stomach pain, gas, or nausea, especially when people don’t take it with a meal. Too much potassium in the stomach can irritate it, and even those with strong stomachs might end up running to the bathroom.
Medical research backs up these regular complaints. According to Cleveland Clinic, gastrointestinal symptoms like abdominal discomfort, diarrhea, and vomiting show up in people taking potassium citrate. The US National Institutes of Health flags similar risks. Mild issues like a weird taste in the mouth or mild cramping can start early and sometimes go away with time, but it’s hard to predict for everyone.
Bumping up potassium levels is essential if a person’s lacking it, but going overboard can send things in the other direction. Potassium stays in balance through the kidneys and the heart relies on the right levels for every beat. If potassium climbs up too high, called hyperkalemia, the heart can act strangely. People might feel weak, confused, or notice an unusually slow heart rate. Medical emergencies linked to high potassium aren’t common for healthy kidneys, but for someone with even mild kidney trouble, that risk increases.
I’ve watched a close friend—already dealing with chronic kidney issues—land in the hospital after adding a new potassium supplement on his own. He didn’t realize the danger until his doctor checked his blood. High potassium can be sneaky. By the time symptoms show, things can get bad quickly.
Anyone with kidney problems, heart failure, diabetes, or chronic dehydration should talk seriously with their doctor before starting or changing a potassium dose. Mixing potassium supplements with other drugs such as ACE inhibitors, diuretics, or NSAIDs can increase the risk of high potassium. People thinking the “natural” solution is safer should remember that too much potassium—whether from pills or food—can spell trouble for some.
Doctors usually order blood tests before and during potassium therapy. Skipping these checks just isn’t a good idea. Take potassium citrate with food or right after a meal and drink plenty of water. This routine helps avoid stomach problems and keeps the kidneys from getting overloaded. People shouldn’t crunch tablets or swallow powder dry, since that can burn the throat or stomach lining.
Health isn’t about blindly trusting every bottle or prescription. It’s about showing up for regular checkups and asking questions, even the simple ones. If something feels off after starting potassium citrate, speak up fast. Doctors want to help, but they need the full story. Getting the dosing right takes participation and attention—not just from the healthcare side, but from the person taking the pill too.
Potassium Citrate Monohydrate plays a big role in labs, medicine cabinets, and even the kitchen if you’re making your own supplements or cleaning products. In healthcare, it often gets mentioned for people who deal with kidney stones or gout. The compound acts as an alkalizing agent, which means it helps keep acids in check in the body. For folks with certain health conditions, like chronic kidney stones caused by high calcium, doctors lean on Potassium Citrate because it helps slow the growth of stones by lowering calcium buildup in urine. The right dosage can make a big difference in quality of life.
If you walk into an American pharmacy and ask for Potassium Citrate Monohydrate, they’ll want to see a prescription. Why? The Food and Drug Administration (FDA) classifies this kind of potassium supplement as a prescription-only product due to the health risks at higher doses. Potassium can become dangerous at the wrong strength, especially for someone with kidney or heart problems; mistakes lead to serious health events like irregular heartbeat, or even cardiac arrest.
In my own experience working with a community pharmacy, I’ve seen patients frustrated by this. Some argue they just want help for leg cramps or to boost their potassium because of diet, but we can’t sell it without a green light from a provider. The FDA sets these rules to stop people from accidentally taking too much. High-dose potassium isn’t something to play with—most people get enough from food, and only those who need intense support from a doctor benefit from large supplement doses.
Some forms of potassium, like the low-dose potassium chloride you find in over-the-counter salt substitutes or multivitamin formulas, are sold without a prescription. In contrast, Potassium Citrate Monohydrate in the doses needed for treating kidney stones stays firmly behind the counter. Online, you might find chemical suppliers offering the raw powder, but those products aren’t regulated for purity or safety, and using industrial sources for health isn’t safe or wise.
Potassium affects so many systems in the body—muscles, heart, nerves—that a misstep with dosage can become a trip to the hospital. It’s not like vitamin C where taking an extra tablet might only upset your stomach. There’s risk even for healthy people, since the body’s potassium range is narrow and the signs of overdose—weakness, numbness, chest pain—sneak up quickly. This is why most reputable health stores won’t carry high-dose potassium citrate for self-use, and most doctors want a blood test before any prescription.
If someone thinks they need extra potassium, getting a simple blood test provides answers fast. For those actually prescribed Potassium Citrate Monohydrate, pharmacists and doctors stay ready to explain how to take it safely, what signs to watch for, and why skipping bloodwork isn’t a good idea. On a bigger scale, better public education about the dangers of unregulated supplement use helps. At the end of the day, a prescription for this compound protects people more than it slows them down.
Doctors often prescribe potassium citrate monohydrate to prevent kidney stones or manage certain urinary conditions. Its job is to reduce the acidity in urine and help keep stones from forming. Those who deal with recurring kidney stones know the frustration—passing a stone feels like a freight train moving sideways. It’s not a medication people take for fun; it’s about managing a real, painful problem.
Mixing potassium citrate with other medications isn’t as simple as swallowing a handful of pills together. Every pharmacy run reminds me how common it is for people to take several different prescriptions each day—sometimes from different doctors, sometimes for years. Mixing certain drugs can turn a helpful medication into a risk, and potassium citrate belongs in that careful category.
Potassium itself affects heart rhythm and the way muscles contract, so it interacts with medication on a basic level. For example, taking ACE inhibitors or certain water pills (like spironolactone) along with potassium citrate can send potassium in the blood too high, causing heart rhythm changes or muscle weakness. Some antibiotics, such as trimethoprim, also raise potassium risk. A person who takes both might never know until the blood test comes back—or something more serious happens.
I’ve seen people surprised by how interactions sneak up. Someone takes potassium citrate for stones, picks up blood pressure medication from another prescriber, and suddenly feels weakness or tingling. These aren’t rare outliers—seniors and people with long-term illnesses run into these scenarios all the time. Doctors and pharmacists spot the red flags, but anyone can get caught off-guard if there isn’t a habit of sharing the full medication list at every visit.
Over-the-counter and herbal products cause surprises, too. A friend once doubled up on potassium by taking a supplement from a health food store, not realizing the prescription medication already covered it. Sitting in the ER with an abnormal EKG cleared up any confusion fast.
Knowledge makes all the difference. Bringing an up-to-date medication list—whether digital or on paper—helps every professional catch problems early. I always suggest marking potassium products clearly: potassium citrate, potassium supplements, even sports drinks loaded with electrolytes. Every bit matters.
Regular blood tests give clues long before symptoms appear. Most clinics have these on routine schedules for people taking medications that change potassium or kidney function. Even if nothing feels wrong, the bloodwork can show a creeping trend.
Open communication counts most. Some people feel embarrassed to ask questions, but experienced doctors and pharmacists know the dangers of keeping silent. Sitting down for a medication review saves lives just as much as the pills themselves.
Technology and teamwork make a difference. Digital health records, pill tracking apps, reminders from the pharmacy—these tools put safety in reach. Training patients to watch for trouble signs, spot ingredient lists, and trust their instincts about side effects turns them from passive recipients into active partners.
No one wants to turn kidney stone prevention into a bigger health issue. Respect for the mix of medications, attention to detail, and support from health professionals keep people out of danger. Every person has a different story, but the lessons about drug interactions never change.
| Names | |
| Preferred IUPAC name | potassium 2-hydroxypropane-1,2,3-tricarboxylate monohydrate |
| Other names |
Tripotassium citrate monohydrate Citrate of potash monohydrate Potassium 2-hydroxypropane-1,2,3-tricarboxylate monohydrate |
| Pronunciation | /pəˈtæsiəm ˈsɪtreɪt ˌmɒnəˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 6100-05-6 |
| Beilstein Reference | 3639072 |
| ChEBI | CHEBI:31624 |
| ChEMBL | CHEMBL1201610 |
| ChemSpider | 26535 |
| DrugBank | DB14544 |
| ECHA InfoCard | 05b65b7c-b4fa-4742-b2ab-a524c156315e |
| EC Number | 206-059-0 |
| Gmelin Reference | 16979 |
| KEGG | C18606 |
| MeSH | D015550 |
| PubChem CID | 23289026 |
| RTECS number | TS7650000 |
| UNII | RU9R6P6S2V |
| UN number | UN3077 |
| Properties | |
| Chemical formula | K₃C₆H₅O₇·H₂O |
| Molar mass | 324.41 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.98 g/cm³ |
| Solubility in water | Very soluble in water |
| log P | -3.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.5 |
| Basicity (pKb) | pKb: 8.5 |
| Magnetic susceptibility (χ) | -7.1×10⁻⁶ |
| Dipole moment | 3.11 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 365.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2190.7 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -3486 kJ/mol |
| Pharmacology | |
| ATC code | A12BA02 |
| Hazards | |
| Main hazards | May cause irritation to eyes, skin, and respiratory tract. |
| GHS labelling | GHS07, Warning, H319, P264, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS). |
| Precautionary statements | Wash hands thoroughly after handling. Do not eat, drink or smoke when using this product. |
| NFPA 704 (fire diamond) | 1, 0, 0, - |
| Explosive limits | Explosive limits: Non-explosive |
| Lethal dose or concentration | LD50 Oral Rat: 5400 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 5400 mg/kg |
| NIOSH | TTD |
| PEL (Permissible) | Not established |
| REL (Recommended) | 340 mg/kg |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Sodium citrate Potassium citrate Citric acid Calcium citrate |