Potassium Dihydrogen Citrate has been around long enough to influence several distinct fields, from food preservation to managing kidney stones. Manufacturers once struggled with sourcing clean, pure acids for buffering and flavoring, turning to natural fermentation or citrus, but those routes didn’t always deliver the batch-to-batch consistency needed for wide industrial use. Chemists eventually hammered out reliable methods for synthesizing the salt, making it an affordable staple not just for labs but for folks dealing with acidity whether in their bodies or their products. The rise of commercial food processing in the 20th century brought attention and investment, and research quickly ramped up as people looked for gentler salts for dietary interventions. It didn’t earn household name status, but those in pharmaceuticals and food science quietly advanced its applications until today, Potassium Dihydrogen Citrate sits in a surprising number of solutions—literally and figuratively. Supply chains have matured, with China and India now manufacturing a significant share of the world’s supply, often tweaking methods to keep up with purity requirements for pharma, food, and water treatment.
As both a buffer and a potassium supplement, Potassium Dihydrogen Citrate pulls double duty. The odorless, crystalline powder dissolves readily in water, so it’s easy to dose and blend. In kidney stone prevention, it alkalizes urine, cutting down on painful calcium stones, while formulators in food lean on it for its tart kick and ability to keep products shelf-stable. Non-GMO, gluten-free sourcing gives it appeal in the expanding world of specialized diets. Reliable performance and predictability in chemical reactions put it on many shelves, often labeled as a “citric acid salt” on ingredient lists. Folks treating metabolic acidosis rely on its potassium and citrate combo—citric acid tames acid buildup, and potassium supports heart and muscle function. Cost has steadily dropped with improved manufacturing, pushing more companies to adopt it over older acidulants or potassium sources like potassium chloride, especially where flavor or application versatility matter.
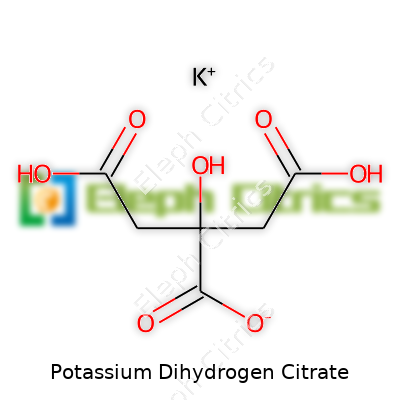
This compound crystallizes into colorless, odorless granules or powder, giving a faintly tart taste. Chemically, its formula is KH₂C₆H₅O₇, blending a potassium ion with a dihydrogen citrate anion. It weighs in with a molecular mass of 230.13 g/mol, and it starts to decompose near 230°C, before reaching its melting point. Solubility in water is robust, but it barely budges in alcohol and stays put in many organic solvents, making water its medium of choice. In solution, it acts as a mild acid with buffering ability, often nudging pH upward in acidic environments. That reliable, stable nature is part of why healthcare professionals choose it—nobody wants fluctuations in a drug mix or beverage stabilizer. It attracts water from the air, so proper storage matters to prevent caking or degradation.
Pharmaceutical and food-grade standards push for potassium dihydrogen citrate with purity upwards of 99%, typically measured by loss on drying, sulfate, chloride, and heavy metal tests using precise instrumental analysis. Products often arrive in lined drums or moisture-resistant pouches, with lot numbers, best-by dates, and country of origin stamped right on the package. Labels on bulk shipments and retail containers must spell out the chemical name, batch code, net weight, and often a warning for restricted medical use. Dietary supplements lay out potassium content per dose, targeting kidney patients, athletes, and anyone with electrolyte needs. Marketers in natural foods sometimes switch the name out for “potassium citrate monobasic,” banking on consumer recognition of potassium’s health value.
Manufacturing Potassium Dihydrogen Citrate comes down to neutralizing citric acid with potassium carbonate or potassium bicarbonate. Operators carefully add hot, concentrated solutions of citric acid to potassium carbonate to kick out carbon dioxide and produce the monopotassium salt. After the fizz dies down, the mixture cools, encouraging crystals to form. Filtration weeds out impurities, and gentle drying produces the finished product. In pharmaceutical operations, extra purification steps—sometimes repeated crystallizations—take things up a notch, tightly controlling for traces of unreacted acids or metal ions. Most facilities run closed-loop systems to recycle waste streams, reflecting a shift toward greener chemical manufacturing. Small tweaks, like pH adjustment and filtration speed, play a major role in yield and quality, pushing manufacturers to find efficiencies batch after batch.
This salt stays pretty steady under most storage and handling scenarios. Add a strong base, and it morphs into dipotassium or tripotassium citrate—each with their own uses and solubility quirks. In acidic conditions, it can offload some potassium, swinging the pH lower and shifting back toward citric acid and potassium ions. Chemists sometimes mix it with other buffering agents in formulations aimed at highly specific pH targets in food, drugs, and cleaning solutions. Heat above 230°C kicks off decomposition, generating potassium carbonate, water, and carbon dioxide—proving much less stable if baked into high-temperature products. It doesn’t tend to form dangerous byproducts, which keeps regulatory barriers lower, compared to more reactive potassium salts.
Look for this compound under “Monopotassium citrate,” “Potassium citrate monobasic,” or the chemical shorthand “KHCit.” Pharmacopoeias call it “Potassium Dihydrogen Citrate” in drug listings, but specialty food suppliers might just say “Potassium Citrate” or “E332(i),” the food additive code in Europe. Sometimes catalogues group it with tripotassium and dipotassium citrate as “citrate salts,” so double-checking with the supplier or a Certificate of Analysis solves any confusion. Large-scale buyers might spot it under proprietary product codes, often ending in -K or referencing its pH of 4 in concentrated solutions.
Being popular in the food and drug world means keeping up with tough safety checks. Workers in plants handle it with gloves and goggles, since dust can irritate eyes or airways. Storage rooms stay dry and cool, reducing the risk of clumping or accidental hydration. The FDA and European Commission grant potassium dihydrogen citrate a “Generally Recognized as Safe” (GRAS) status for foods, but anything ending up in medicines needs to meet strict pharmacopoeial benchmarks for impurities, especially heavy metals. Facilities log each lot for traceability, keeping recall risks low. In the lab, extra safety measures come into play during heating or pH adjustment, since even a seemingly gentle buffer can become corrosive at high concentrations. Ventilation matters as carbon dioxide can evolve if strong acids or bases interact with spilled material.
Hospitals and clinics prescribe potassium dihydrogen citrate to both prevent and dissolve certain types of kidney stones. Urologists count on it to bump up urinary citrate, creating a less hospitable environment for stone formation, which saves patients recurring ER trips and surgeries. Nutritionists and supplement makers add it to products targeting people at risk of potassium deficiency, blending it with sports drinks and meal replacements for athletes, those on diuretics, or anyone with electrolyte imbalances. Beverage companies appreciate its tart flavor and its ability to counteract alkaline aftertaste, using it to balance sweetness in juices or soft drinks. Food manufacturers use it for preservation, especially in low-salt and processed foods, as it controls both acidity and microbial growth. Industrial settings use it for water softening, where it binds calcium and magnesium, extending the life of boilers and cooling systems. Green cleaning brands add potassium dihydrogen citrate as a biodegradable buffer or chelating ingredient, reducing hardness in eco-friendly detergents.
Labs devote resources to new synthesis methods aimed at better yields, lower costs, or reduced waste. Analytical chemists spend time tweaking high-performance liquid chromatography (HPLC) checks to pick out impurities at parts-per-million levels. In pharma, teams trial slow-release tablets and advanced blends to stabilize active drugs using potassium dihydrogen citrate. Dietary researchers investigate its long-term effects on heart health, kidney function, and bone density, especially as low-salt diets become more widespread. Food engineers experiment with blends that maintain flavor and shelf life but dial back sodium, looking to potassium dihydrogen citrate as a key replacement. Startups in the clean tech world ask how to use its chelating power in water treatment, pushing for more resilient, less toxic chemical cycles in industrial applications. These efforts keep the compound in the spotlight for grant funding, regulatory review, and market expansion.
Toxicologists keep a close eye on intake limits. Excess potassium—no matter the salt—can upset heart rhythms, especially for older adults or those with kidney issues. Medical literature flags side effects like muscle weakness or digestive upset at high doses, so products list potassium content clearly. Animal studies, where high-dose feeding occurs, show low acute toxicity but hint at chronic risks if balance isn’t respected. National agencies cap daily potassium intake in medicines and supplements, prompting manufacturers to tailor dose forms and warnings. In rare cases of massive overdose, potassium dihydrogen citrate acts as a corrosive, causing internal irritation. Standard treatment in emergencies relies on dilution, electrolyte balancing, and close heart monitoring. Pharmacies and clinical staff screen patients on potassium therapy for contraindications, especially if the patient takes ACE inhibitors or certain diuretics.
With more patients worldwide managing kidney stones and heart conditions, hospitals and drugmakers bet on steady growth for potassium dihydrogen citrate. Global shifts toward cleaner energy and less wasteful chemical production drive research into new green synthesis routes and recycling methods. The food industry banks on low-sodium diets gaining traction, bringing this potassium salt further into the mainstream for flavor and preservation. Demand will likely spread into cosmetics and agriculture once safe and effective formulations emerge. Ongoing toxicity research pushes for tighter safety margins, smart dosing strategies, and clear labeling. As more of the world pays attention to nutrition and sustainable chemistry, potassium dihydrogen citrate sits at a new crossroads, ready for broader acceptance and tougher scrutiny—all signs point to a busy decade ahead for this once-niche ingredient.
Potassium dihydrogen citrate shows up in medicine cabinets far more often than people realize. Doctors use it to treat kidney stones that may form from too much uric acid or certain kinds of calcium. In my own circle, I’ve watched friends try to pass small stones, and it’s always uncomfortable. The pain pushes them to look for ways to keep stones from coming back. They end up talking with a physician about taking potassium dihydrogen citrate, because it raises the citrate level in the urine. Higher citrate helps prevent stone formation by binding with calcium, so there’s less risk of solid crystals creating trouble down the line. I’ve seen folks relieved by this solution, grateful for fewer trips to the emergency room.
Doctors also turn to potassium dihydrogen citrate for another job: Replacing lost potassium in folks who get low levels because of diuretics, diarrhea, or other causes. Potassium supports muscle control and a healthy heart rhythm. Without enough, people feel weak or even dizzy. Potassium dihydrogen citrate helps correct the balance, letting muscles and nerves work like they should. It tastes a bit tart, so it often goes into pill or powder form rather than as a drink.
Truth is, potassium dihydrogen citrate doesn’t only belong in prescription bottles. Food companies use it for a handful of reasons. It acts as a stabilizer and a preservative in sodas, sports drinks, and even some desserts. Food scientists lean on it to control acidity in products. Drinks with a tart bite—like lemonades—sometimes need a little help in the background to keep the flavor consistent. Potassium dihydrogen citrate really shines in this job because it does not bring a salty taste, which potassium chloride tends to do.
Home cooks may not reach for this directly, but the food industry picks it for low-sodium recipes and items for people watching their salt intake. It supplies potassium, which supports heart health, and at the same time, helps balance flavors in processed foods or powdered mixes.
Potassium dihydrogen citrate plays an unglamorous but important part in chemical labs and manufacturing. Lab technicians use it as a buffer. During chemical reactions or when running samples through a machine, acidity needs to stay in a safe range. Potassium dihydrogen citrate helps set and maintain that sweet spot, so experiments behave as expected.
Water treatment facilities look at chemicals like this one for softening water or balancing pH. Soft water protects machinery, pipes, and even improves the texture of clothes and hair washed in it. It also helps medications designed for people who rely on dialysis—water used in these machines must stay extremely pure and balanced, and chemicals like potassium dihydrogen citrate make that possible.
One challenge with potassium dihydrogen citrate comes from keeping the dose just right. Too much potassium in the body stresses the kidneys, especially for people whose organs already struggle. Doctors and food makers have to keep an eye on amounts. Better public education about how much potassium healthy adults need—and when to avoid it—would help people pick products more safely. Clearer labels and guidance from pharmacists go a long way too. A little awareness widens the safety net, helping people get the benefit without surprise side effects.
Potassium dihydrogen citrate often comes up in conversations about kidney stones or gout. Doctors sometimes hand out these pills to raise potassium levels or to make urine less acidic. With more folks being told to watch what goes in their bodies, it feels right to take a plain look at what happens after swallowing this crystal white powder mixed in water or packed into a tablet.
Most people who take potassium dihydrogen citrate for the first time expect it to help with what ails them. Still, the body has its own way of telling you when things aren’t just right. Stomach upset lands near the top of complaints. Nausea shows up. Vomiting, stomach pain, and diarrhea might send you hurrying to the bathroom or looking for crackers to settle your stomach. Doctors warn against lying down right after taking your dose—doing so increases your chances of heartburn.
I’ve seen people brush off these gut troubles, thinking they’ll pass. Sometimes they do, but sometimes the symptoms hang around long enough to make someone quit the medication. Changing the dose, drinking more water, or eating a snack with your pill can sometimes ease the symptoms.
The bigger worry has to do with potassium itself. Our kidneys usually work hard to flush out what we don’t need, but too much potassium in the blood (hyperkalemia) sets off alarms. Muscles can cramp or feel weak, and heart rhythms can go haywire. This risk jumps up in people with kidney disease, those taking drugs like ACE inhibitors, or folks with adrenal problems.
One big study from the Annals of Internal Medicine years ago showed that potassium disturbances often come without much warning. Tiredness, tingling, or a funny feeling in the pulse sometimes pop up, but it’s easy to overlook them. Poor kidney function raises this risk so quickly that most doctors want to see regular blood tests if you’re on this supplement for the long term.
People over 65, children, and those taking heart medications seem to walk a thinner line. That’s not scaremongering—it’s just what the numbers show in clinical reports. A 2023 review in the Journal of Clinical Pharmacy dialed in on the increased risk for older adults, especially those on blood pressure pills or diuretics.
Most troubles linked to potassium dihydrogen citrate target the gut or kidneys. Drinking plenty of fluids, splitting doses, and taking the medicine with meals often makes a difference. Regular check-ins with a doctor, plus blood work, catch any imbalance before it tips over into the danger zone. Pharmacists and dietitians also help in sorting out whether a particular person could handle this supplement or needs to look for something milder.
As someone who’s seen both patients who breeze through potassium supplements and those who run into side effects, steady communication with health workers always beats guesswork. No pill is risk-free, but careful steps make an enormous difference with this one. For anybody thinking about potassium dihydrogen citrate, a real talk with your health team fits better than rolling the dice and hoping for the best.
Over the years, I’ve seen how small details in handling chemicals can build up to big consequences, both in labs and at home. Potassium dihydrogen citrate, often found in dietary supplements and medical preparations, calls for the same careful respect that goes into handling any substance meant for human use. Storage isn’t just about following rules on a data sheet; it protects the product’s stability and the safety of everyone nearby.
Potassium dihydrogen citrate thrives in a cool, dry spot. Warm and humid air can set off a chain reaction. The powder starts clumping, and moisture can spark chemical changes that weaken its benefits or even create new hazards. I’ve lost more than one bottle to a bathroom cupboard in summer—one day you have fine granules, the next, a brick.
A cabinet away from direct sunlight and away from any sources of water keeps things steady. If the room swings between cold and hot, it’s better to use a space with climate control. Basements sometimes tempt people for chemical storage because they’re out of the way, but damp air down there can cut shelf life short and turn a good ingredient useless.
The container matters just as much as the room. I always use tightly sealed, food-grade plastic or glass containers. Manufacturers usually ship potassium dihydrogen citrate in screw-top bottles or heavy-duty pouches. Keeping the original packaging helps because it’s designed to protect against air and moisture. After opening, don’t swap out containers unless absolutely necessary—if you do, pick one that seals fully and leaves no room for spills or air leaks.
Avoid storing it in paper or thin plastic bags. Paper soaks up water, and thin bags rip too easily for anything that you’re ingesting or using in careful doses. If kids or pets have a knack for poking around the house, use a high, locked drawer because accidental spills are a headache you don’t need.
I think one of the most neglected steps lies in what sits around the potassium dihydrogen citrate. Mixing this powder with strong acids or bases kickstarts unpredictable reactions. For example, storing vinegar or cleaning supplies on the same shelf can spell trouble. Any cross-contamination can reduce purity or create minor hazards, so always give chemicals their own space.
Storing potassium dihydrogen citrate long past its expiration isn’t a clever thrift trick. Manufacturers place those dates for a reason—a chemical slow fade, not a sudden spoil. Mark expiry dates on the bottle or a nearby list, and toss anything past its prime responsibly. Municipal rules often demand disposal at a facility rather than a household trash can. Look into those guidelines before you’re left holding a bottle you can’t throw out.
From a personal angle, don’t let busy days push chemical storage into the background. Simple habits—checking containers, marking dates, cleaning shelves—make a world of difference. Potassium dihydrogen citrate isn’t intimidating if treated with a blend of routine care and knowledge. Every time I follow these steps, peace of mind kicks in, and lost product becomes a thing of the past.
Potassium dihydrogen citrate usually comes up in a doctor's office for folks dealing with kidney stones or certain kidney conditions. It helps reduce the formation of stones by making urine less acidic. The body struggles without the right amount of potassium, but too much can harm the heart and nerves. That’s why many pharmacists and kidney doctors keep a close eye on dosing.
Doctors usually adjust the dose based on blood tests and symptoms, not just the bottle’s label. The usual starting point for adults falls somewhere between 10 and 30 milliequivalents (mEq) a day, split into three doses. Sometimes, people require more, sometimes less. Each person’s dose depends on kidney function, how much potassium they already have in their blood, and how well their body tolerates the salt. The kidneys filter out excess potassium—unless they are sluggish, which makes potassium build up fast.
Some folks skip prescription advice and look for answers online or start supplements from a vitamin shop. This shortcut can backfire. Blood potassium rises before symptoms show up. Muscle weakness or heart rhythm changes might appear only after things have drifted far off course. According to the National Institutes of Health, keeping blood potassium in check can literally be the difference between life and death for people with heart or kidney problems.
A key piece often overlooked is continuous monitoring. Doctors run blood tests to check potassium and kidney function every few months for long-term users. When a person starts or stops other meds—think water pills or blood pressure pills—those can push potassium to unsafe levels. Even something as common as eating lots of bananas or using salt substitutes can sneak in extra potassium. Health workers stress not mixing over-the-counter potassium products with prescription sources unless instructed.
Years ago, I watched a relative wrestle with chronic kidney stones. She followed advice from her nephrologist, who kept her on potassium dihydrogen citrate for nearly two years. Every dose change linked to her lab test and diet—never just a random guess. Water intake mattered almost as much as the prescription. One winter she caught the flu, got dehydrated, and ended up in the ER with sky-high potassium, all because she hadn’t adjusted for her lost fluids. That taught our family doctors don’t hand out these meds like multivitamins.
If there’s one thing that sticks, it’s this: follow medical guidance. Doctors set potassium citrate doses to prevent kidney stones and preserve normal blood chemistry. Most adults fall into the 10–30 mEq three times per day range, but staying in touch with a healthcare provider keeps things safe. Get labs on time, report new symptoms fast, and avoid mixing this salt with other potassium sources unless given a green light. Trust real experts and lean on experience shared by medical professionals, not rumor or social media advice.
Access to clear information empowers patients but can’t replace blood test results or conversations with a clinician. Reform in how supplements get sold could stop accidental overdoses, but until then, responsible use rests with the prescriber and the patient together. The safest path means routine checkups, honest reporting, and respect for the risk potassium brings when it lands in the wrong hands or gets used outside medical supervision.
Potassium dihydrogen citrate helps people manage certain medical conditions, including kidney stones and some urinary tract issues. Doctors sometimes suggest it for people with low potassium or to prevent stones from forming again. This mineral does important work controlling fluid balance and supporting muscle and nerve function. In some pregnancies, potassium levels can drop, making it tempting to reach for supplements or medications that include potassium salts.
Big organizations like the FDA, the American College of Obstetricians and Gynecologists (ACOG), and the World Health Organization always stress one point: before taking anything during pregnancy or breastfeeding, talk to your doctor. As potassium dihydrogen citrate is classified as a prescription medicine in most places, doctors typically prescribe it only after running tests and deciding the benefits outweigh the risks. It hasn’t received the kind of research funding that common prenatal vitamins get, so long-term safety data during pregnancy and breastfeeding remains limited.
A woman’s body handles minerals differently during pregnancy. Blood volume increases, hormones change, and the kidneys work harder. All these changes affect how the body uses and gets rid of potassium. Too much potassium, called hyperkalemia, triggers muscle weakness, irregular heart rhythms, and—if left unchecked—can lead to more serious problems. Unexpectedly low potassium, on the other hand, brings cramps, tiredness, and sometimes dangerous changes in heart rhythm. Pregnant women often walk a fine line between the two.
With limited controlled studies, no one can say for certain that potassium dihydrogen citrate won’t cause harm to a developing baby or a breastfeeding infant. Case reports exist of potassium supplements leading to high blood potassium, especially in women with preeclampsia or kidney complications—a population that’s already vulnerable. Breast milk transfers many nutrients and minerals from mother to infant. Small changes in a mother’s mineral intake reflect quickly in breastfed newborns, who are much more sensitive to excess potassium.
I have seen friends, family, and patients face decisions about supplements in pregnancy. Always, the safest route meant talking to a trusted healthcare professional. Doctors usually order lab tests before starting potassium supplements, monitor closely, and adjust doses based on need. Pregnant and breastfeeding women deserve tailored care, not one-size-fits-all answers. At the pharmacy, over-the-counter potassium citrate powders and pills might look harmless, but real risks lurk when self-prescribing.
If a doctor diagnoses low potassium during pregnancy or lactation, medical guidance can make sure you get the right dose, in the safest form. Diet often provides the better solution. Foods like bananas, sweet potatoes, beans, and yogurt pack potassium naturally without the risk of overdosing. Plenty of fluids also help support kidney health and reduce the chance of stone formation. Anyone considering supplements should ask: is there a real medical reason, and has a professional signed off on it?
Science could stand to learn more about potassium supplements in pregnant and breastfeeding women. Until those answers arrive, no one should self-treat with potassium dihydrogen citrate in these vulnerable times. Staying informed and keeping an open line of communication with a knowledgeable healthcare provider supports the healthiest start for both mother and baby.
| Names | |
| Preferred IUPAC name | potassium 2-hydroxypropane-1,2,3-tricarboxylate dihydrogen |
| Other names |
Monopotassium citrate Potassium citrate monobasic Monobasic potassium citrate |
| Pronunciation | /pəˈtæsiəm daɪˈhaɪdrəʤən ˈsɪtrət/ |
| Identifiers | |
| CAS Number | 866-84-2 |
| Beilstein Reference | 1901386 |
| ChEBI | CHEBI:131370 |
| ChEMBL | CHEMBL1201650 |
| ChemSpider | 159466 |
| DrugBank | DB14517 |
| ECHA InfoCard | ECHA InfoCard: 003-008-00-5 |
| EC Number | 01-2119457026-42-0000 |
| Gmelin Reference | 162039 |
| KEGG | C13702 |
| MeSH | D020146 |
| PubChem CID | 6433882 |
| RTECS number | TT1510000 |
| UNII | RU7T31AP0T |
| UN number | UN3077 |
| Properties | |
| Chemical formula | KH₂C₆H₅O₇ |
| Molar mass | 306.39 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.984 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 3.1 |
| Basicity (pKb) | pKb ≈ 11.6 |
| Magnetic susceptibility (χ) | \-53.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.430 |
| Dipole moment | 4.4 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 234.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1618.1 kJ/mol |
| Pharmacology | |
| ATC code | B05KA2 |
| Hazards | |
| Main hazards | May cause irritation to eyes, skin, and respiratory tract. |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: "P264, P270, P301+P312, P330 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 (oral, rat): 5400 mg/kg |
| LD50 (median dose) | 3300 mg/kg (rat, oral) |
| NIOSH | GTZ35 |
| PEL (Permissible) | Not established |
| REL (Recommended) | Not more than 2.0 g daily |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Monopotassium phosphate Tripotassium citrate Potassium bicarbonate Potassium carbonate Citric acid |