Potassium hydrogen malate stands on the shoulders of centuries’ worth of tinkering and curiosity in both chemistry and food science. The use of malic acid traces back to early studies of apples and other tart fruits—way before the pure salt versions hit scientific shelves. Folks started isolating and modifying acids as curiosity grew around food preservation, winemaking, and the hunt for gentler acidulants. As potassium-based salts took off in medicine and nutrition, chemists noticed the unique qualities that potassium malates brought to the table. By the late twentieth century, as technology improved, production ramped up, and its applications in areas like food, pharmaceuticals, and lab chemistry spread far and wide. The story of potassium hydrogen malate isn’t just about one discovery. It’s a slow build, shaped by practical needs and a steady march of research.
Potassium hydrogen malate, often popping up under names like monopotassium malate or potassium acid malate, tends to show its face in food ingredients lists, nutritional supplements, and technical reagents. In food, it acts as an acid regulator, tangy flavor enhancer, and buffer, supporting stable product formulas. In pharmaceuticals, it delivers potassium without the punch of common alternatives like chloride or citrate. Whenever a technical recipe wants a mild acid without a metallic taste or high sodium, the monopotassium version steps up. It’s the quiet helper—never as glaring as the more famous citrates or phosphates, but useful for its kind touch.
Anyone who’s worked with potassium hydrogen malate recognizes its white crystalline powder—slightly hygroscopic, easy to dissolve in water with minimal residue. The malate anion’s two carboxylic groups give it flexibility. You’ll spot it melting at temperatures just above 150°C, holding its own in mildly acidic to neutral solutions. One carboxyl group is neutralized and the other is still holding on to an acidic hydrogen, so its pH sits around the 4 to 5 mark for a 1% solution. Potassium’s presence adds nutritional value, while keeping the compound’s taste profile pleasant. Handling it in the lab or food plant usually feels like handling table salt—no aggressive odors, no staining, and only a faint tartness when tasted (not that I ever tried, of course). In water, it breaks apart smoothly into potassium ions and hydrogen malate, playing nice with enzymes and other food acids.
Labels for potassium hydrogen malate products should always flag its dual identity—potassium and malic acid. Food coders might write E351 or call it monopotassium malate. For pharma and technical packs, certificates explain purity (often 98% or greater), residual moisture, and heavy metal limits. Specifications from suppliers typically guarantee low sodium levels, standardized particle size for fast dissolution, and compliance to food or pharmacopeial standards. Reliable suppliers make sure to state possible allergens, cross-contamination points, and shelf-life, which usually runs a couple years in dry, sealed storage. On finished product ingredient lists, you might spot it simply as “potassium malate”—but savvy formulators double-check labeling for legal quirks, especially in strict regulatory settings.
Most potassium hydrogen malate gets made by neutralizing a solution of malic acid—sourced either from apples, as in olden days, or commercially fermented via microbial strains using glucose—then tossing in potassium carbonate or potassium hydroxide. The reaction releases carbon dioxide and water, and if done carefully, ends with one potassium for every malic acid and leaves a single carboxyl group untouched. After mixing, the solution goes through filtration to clear out insoluble matter, then concentrated and crystallized as the solution cools. To make the pure stuff, manufacturers wash the crystals and dry them in warm air. It’s simple enough for college labs but requires care on an industrial scale to avoid contamination and get the particle size right for each use. Each lot’s purity and physical look gets tested before hitting the market, since the smallest mishap could mess with taste or function.
Potassium hydrogen malate isn’t just a background player. Its free carboxyl group means it can act as a buffer, mop up excess bases or acids, and participate in gentle chemical reactions. When heated with strong bases, it transforms further to potassium malate—a fully neutralized salt—useful for more basic applications. Mixed into a reaction with calcium or magnesium salts, it forms sparingly soluble malates, sometimes drawing out minerals from water solutions or acting as a chelating agent in bio-processes. On the research front, tweaking the ratio of potassium to malic acid lets scientists control release rates in pharma preps or tinker with acidity in technical food blends. There’s a lot of room for creative chemistry here, with the malate structure offering both stability and a chance for further modification if the case calls for it.
Ask around different industries and you’ll hear potassium hydrogen malate called monopotassium malate, potassium acid malate, E351 in food codes, or even simply “potassium malate” in looser regulatory settings. Chemical catalogs sometimes link it to CAS number 585-05-7. Pharma suppliers might stick with the precise monopotassium name. International product names change a bit depending on translation quirks, but it’s always the same salt at its heart—potassium paired up with the singly protonated malate anion.
People don’t face much risk working with potassium hydrogen malate—certainly less than with stronger acids, caustic alkalis, or heavy metal salts. Safe handling involves eye protection and basic dust masks if you’re around a lot of powder, since it can irritate the airways in sensitive folks. Material Safety Data Sheets point out the low acute toxicity and urge precautions only for bulk handling or mixing with other strong chemicals. Food-grade and pharma-grade batches go through purity checks, and good manufacturing practice stays critical. Bigger risks show up in cross-contamination or if vats get overloaded and fermentation goes off the rails. In the decade or so I’ve followed food processing issues, very few recalls or accidents ever came from potassium hydrogen malate itself—usually the trouble comes from poor oversight or mislabeled containers. Standard hygiene, temperature control, and equipment checks keep both the workplace and product safe.
Applications for potassium hydrogen malate bridge a lot of worlds. In the food industry, it offers an easy way to add potassium and a light acid note to candies, drink mixes, baked goods, and dairy. Soft drink producers like how it adjusts pH without ruining flavor profiles, so it pops up in new sports drinks and juices. Nutritionists and supplement developers include it for potassium fortification, especially when they want to avoid the bitter bite or high cost that comes with potassium gluconate or citrate. On the technical side, potassium malate is handy as a buffering agent in fermentations, lab protocols, and diagnostic reagents. Some pharmaceuticals use it for slow-release potassium, helping patients balance electrolytes. Winemaking draws on malates during malolactic fermentation, controlling acidity and helping certain grape profiles. Whenever you need an acidulant that won’t overshadow delicate flavors or alter mineral balance, this compound often becomes a go-to.
Recent years have seen more attention on plant-based supplements and functional food ingredients. Researchers dig into potassium hydrogen malate’s antioxidant actions, bioavailability, and support for metabolic pathways connected to the citric acid cycle. Nutrition science sees promise here, especially for groups needing balanced potassium intake. Technical innovations include smarter ways to recover and purify the salt from fermentation broth, or even engineer microbes to crank out higher yields. Food technologists keep pushing boundaries—looking for new blends and uses that tap into the gentle acidity and unobtrusive mineral profile. Some pharma studies chase slow-release tablets and new electrolyte therapies, hoping this gentle salt can break new ground for patients with heart or kidney problems. From what I’ve seen at conferences and in journals, curiosity is far from running out—there’s always a new hypothesis about malate’s cellular roles or a better way to put its properties to use.
The research on potassium hydrogen malate’s safety is reassuring. Both potassium and malic acid show low toxicity when consumed in amounts typical for food or medical use. Malate, produced naturally in the body during energy metabolism, clears out easily unless someone has a rare metabolic defect. Potassium presents more risk at high doses if a person’s kidneys struggle, since hyperkalemia can cause real trouble. Multiple food safety authorities—like the European Food Safety Authority—have reviewed the compound and found no cause for concern in usual applications, setting generous daily intake limits. Long-term feeding studies in rodents show no negative effects on organs, reproduction, or growth. That said, the usual caution holds: don’t toss massive amounts into supplements or meals. Products targeting kidney or cardiac patients should flag total potassium content and avoid accidental overdoses. In a decade of food safety reading, rarely does potassium malate come up as an offender.
The future looks bright for potassium hydrogen malate, as demand for clean-label food acids and potassium-rich supplements only seems to grow. Its friendly taste and ease of handling mean it’s well placed for next-generation nutrition products—sneaking potassium into low-sodium snacks, shakes, and beverages without those off-flavors that other salts drag along. Consumer watchdogs and food safety agencies push for clearer labeling, so traceability and data transparency will get sharper year on year. Researchers hunt for new fermentation strains, aiming at both lower cost and even cleaner production (think non-GMO, organic-certified). Regulatory bodies may revisit intake limits as more supplements enter the market. Product developers test malate combinations in athletic recovery drinks, fortifying plant milks, or even buffering new pharmaceuticals. It’s clear that as dietary needs get more specific and tastes more refined, potassium hydrogen malate will keep carving out space as a quietly valuable, reliable ingredient for years to come.
Chances are, if you’ve opened a package of baked goods or mixed a powdered drink, you’ve come across potassium hydrogen malate. It’s a food additive that often flies under the radar because it hides behind technical language and complicated ingredient lists. In truth, this compound keeps things tasting fresh, maintains a pleasant mouthfeel, and stops flavors from turning sour. I remember as a kid marveling at how powdered pops turned into sparkling drinks—unaware that behind the scenes, potassium hydrogen malate made the magic possible.
Its most important job in foods involves acidity. Many processed foods rely on acid regulators to balance taste and preserve texture. Too much acid, and a dish is harsh; too little, and it’s flat or bland. Potassium hydrogen malate steps in to keep that sweet-spot pH, brightening tart fruit flavors or smoothing the sharpness out of sauces and candies. In my pantry right now, I’ve got drink mixes and flavored vitamin chews—both owe their appeal to careful acid adjustment, with this compound in the mix.
People reach for mineral supplements more often now, and one mineral that deserves more attention is potassium. Heart health depends on stable potassium intake. My doctor once told me low levels are bad for nerves, blood pressure, and muscles. Not everyone gets enough from food, especially with busy lifestyles, and supplements fill that gap.
Potassium hydrogen malate is handy here, dissolving well and carrying a neutral taste. Compared to potassium chloride, which tastes bitter or salty, it’s friendlier to the palate in drink mixes or effervescent tablets. If you check the labels of electrolyte powders or sports drinks, this ingredient pops up, quietly helping keep runners and gym-goers hydrated and healthy.
Adding potassium salts like this one is more than just about taste. The world faces a sodium overload. Many snacks and processed foods push blood pressure up by being heavy on salt. Swapping some sodium out for potassium benefits the heart. Food scientists turn to potassium hydrogen malate as a way to boost potassium content subtly, giving heart health a boost without ugly flavors or weird aftertastes.
Better-for-you foods aren’t only about cutting what’s bad; they add what helps. It’s good to see more companies tweaking their recipes toward this balance. History shows that small ingredient shifts can lead to public health success—much like folic acid in bread dropped rates of birth defects. Substituting sodium salts with potassium ones looks like a smart step for the next generation of everyday products.
Consumers deserve to know what they’re eating. Too often, ingredient lists get cluttered with unfamiliar chemicals, leaving people guessing about what they’re putting in their bodies. Honest labeling and outreach about these additives reduce confusion and suspicion.
There’s a simple fix: food makers and regulators could use plain language alongside chemical names on packaging, so everyone from parents to students learns why these ingredients matter. Potassium hydrogen malate adds value to a wide range of foods and supplements, and public trust grows when companies make their benefits clear without hiding behind jargon.
Potassium hydrogen malate pops up in more food products than most people realize. It acts as a food additive, helping control acidity or extending the shelf life of what’s on grocery shelves. I see its name in ingredient lists when my family picks up pre-packaged sauces or flavored waters. Most folks aren’t thinking about chemistry at the dinner table, but a name like “potassium hydrogen malate” can raise eyebrows.
Researchers and food regulators have dug into this compound. Bodies like the European Food Safety Authority and the U.S. Food and Drug Administration allow its use, calling it “generally recognized as safe” (GRAS) when it shows up in reasonable quantities. The human body deals with both potassium and malic acid—two ingredients formed by breaking down the compound. Our kidneys balance extra potassium, and we naturally turn malic acid into energy during normal metabolism. This chemistry happens without us noticing, every day.
Eating too much potassium can trouble some people, mainly those with kidney issues who can’t get rid of it easily. Most healthy people handle reasonable amounts without trouble. A food additive like potassium hydrogen malate usually puts just small amounts of these chemicals into a meal. So for most people, there’s no risk of hitting dangerous potassium levels simply from eating food with it included.
Not everyone’s body works the same. Anyone with advanced kidney disease or certain heart conditions sometimes needs to closely monitor potassium intake. Doctors often say the same about bananas, potatoes, or spinach, foods known for high potassium and good nutrition. With food additives, clear labeling helps. Knowing what’s in your food lets you make smart choices—something people with medical conditions rely on every trip to the store.
The other piece of the equation comes from consumer awareness. Open conversations with healthcare providers make a real difference. I’ve sat in a doctor’s office with a family member who needed to ask about potassium levels because of a prescription medication or blood pressure issues. Label transparency, regular checkups, and guidance from real professionals go a long way to making these ingredients safe for everyone.
Regulators review food additives based on lab data, animal research, and, if available, long-term human experience. Potassium hydrogen malate has made the cut in the U.S. and Europe. The real test, though, plays out in daily meals. If any ingredient causes new allergies or sensitivities, reporting side effects and gathering data remains crucial. History shows our food system responds best to regular updates—science doesn’t stand still, and neither do regulators.
It’s tempting to want “chemical-free” food, but the reality is more nuanced. Even an apple or orange holds chemical compounds, most of them safe. Potassium hydrogen malate sits on the same spectrum. Big, global agencies watch for evidence of harm, stepping in when something tips the balance. In my home, I check labels and mix up meals, making room for fresh whole foods while accepting that safe, regulated additives play a role in modern convenience.
Labeling rules can always improve, especially for people with specific health concerns. Public education helps cut through worry and confusion about long ingredient names. Questions about food safety deserve straight talk, not alarm or hand-waving. With the right information, people make confident choices for themselves and their families. Potassium hydrogen malate keeps its place on the menu for now—part of a bigger food safety story grounded in evidence, not guesswork.
Potassium hydrogen malate often shows up as a food additive and in dietary supplements. Our bodies use potassium to help nerves fire and muscles contract, while malate, coming from apples and other fruits, plays its part in the Krebs cycle, fueling cell energy. Folks sometimes ask doctors about this compound, especially people with kidney concerns or those managing their blood pressure.
Every supplement or added ingredient carries a double edge—potassium hydrogen malate isn’t special here. The big concern is too much potassium, what the medical field calls "hyperkalemia." Heart rhythm goes out of sync, tiredness sets in, and a person can feel muscle weakness. In my years talking with pharmacists and nurses, they keep a sharp eye on people who have kidney issues because kidneys keep potassium in balance. Healthy kidneys usually toss out what the body doesn’t need, but if those filters slow down, potassium climbs.
Stomach upsets show up for some users: bloating, cramps, and, on rare days, diarrhea. Most people don’t think much about taking a food additive with a name like this, but if you eat a lot of "potassium-fortified" foods or take several supplements, you raise your risk without even trying. I remember a case in my own circle: a friend on an unusual diet landed with stomach trouble after stacking "natural" electrolyte boosters. He had no idea the additives in his drinks combined with pills.
Researchers have studied potassium in many forms, but potassium hydrogen malate hasn’t appeared in many large human trials. Most advice comes from case studies or what happens with other potassium salts, like potassium chloride. Medical journals mention allergic reactions as rare but possible. I’ve seen some people assume "malate" means "made from apples so it’s safe," but that’s too simple. The source does not always predict how a compound will act inside the human body, especially once it starts interacting with medication.
Drug interactions can sneak up, too. People taking blood pressure pills, especially ACE inhibitors or potassium-sparing diuretics, can push their levels higher without meaning to. Digging through FDA databases and health sites, I found real stories of hospital visits from supplement overload. Sometimes even a strong athlete can see side effects after chasing electrolyte balance without enough guidance from a doc or trainer. The lesson here: people need real-world, practical advice, not just chemistry facts.
Instead of guessing, people should check food labels and talk to a doctor before starting supplements, especially if a kidney problem, heart issue, or medication comes into play. If cramps, tingling, or unusual tiredness come up, those warning signs shouldn’t be ignored. It helps to stick to recommended amounts and avoid doubling up just for the promise of more energy or quicker recovery.
Companies can do their part with honest, clear labeling. Health providers should flag risks of stacking pills with potassium-rich foods. By making small changes and paying attention, most people can avoid the rare but serious side effects that put potassium hydrogen malate in the news.
Walking into any lab or food production space, you’ll spot shelves lined with containers labeled in all manner of ways—some more faded than others, some with contents clumped from exposure. These details seem small until you realize they can directly affect product safety, research accuracy, or even employee wellbeing. Potassium hydrogen malate, often used in foods and sometimes in scientific settings, isn’t immune to these risks. If moisture sneaks in, the compound can start clumping, changing how it measures out in recipes or experiments. Nobody wants a pie crust ruined, or a chemistry trial botched, just because a jar wasn’t tightly closed.
The key lies in remembering this compound is fairly stable, but it’s not invincible. Water in the air turns into trouble. That means a tightly sealed container—preferably something with a screw top, not just a snap-on lid. Sticking with a dry, cool spot makes a big difference. Fluctuating temperatures speed up the breakdown of many powders, including this one. Sunlight heat or a poorly insulated storage area means extra clumping risk and a shorter useful life.
I’ve seen kitchen teams lose whole batches of baking ingredients from careless storage. It’s easy to put a jar back wherever there’s space, but high humidity areas, like above a stove or under a sink, aren’t ideal for potassium hydrogen malate. My own habit has been to use airtight glass jars and keep them on shelves away from heat sources or water pipes. In labs, this goes for the desiccator cabinet—those silica packs in the jar do a lot more good than most folks think.
Another trouble spot comes from poor labeling or open access. Food-grade compounds look a lot like laboratory reagents sometimes. Potassium hydrogen malate sounds safe, but cross-contamination still happens. A faded label doesn’t warn a baker who’s grabbed the wrong jar. I’ve run into situations where an intern in a lab scooped from the wrong powder, simply because the marking rubbed off. Permanent marker on freshly wiped glass, with big easy-to-read letters, saves money and prevents accidents.
Even with solid storage methods, life gets busy and old habits creep in. The best solution: build routines. Make it policy to check seals, check labels, and check placement every so often. I know a bakery manager who schedules ingredient audits every Friday. In my experience, labs that assign one person responsibility for the chemical shelf see fewer mix-ups and less wasted material. Posting a sign above the storage area with simple reminders helps catch these issues before they cost a day’s batch—or worse, impact safety.
Regulations and safety standards already call for dry, well-ventilated conditions. Those rules reflect real problems people see every year, from spoiled product to actual injury. Making a habit of proper storage isn't just legal compliance, it's about protecting people and the effort put into every experiment or recipe.
People who care about their food and their diet know the value of minerals. Potassium deserves a special mention. It keeps nerves firing, helps muscles contract, and stops blood pressure from shooting up. Potassium hydrogen malate appears in some nutrient supplements. It pops up in workouts, and doctors sometimes turn to it for kidney stones or low-potassium problems. But popping any supplement or pill means paying attention to the other things you take. In the world of medications, mixing things up can set off new problems.
Doctors have known for a long time that potassium in large doses can clash with some drugs. The body likes balance: too much potassium, and the heart sends warnings. Mixing potassium supplements—even ones based on malate—together with certain blood pressure drugs, such as ACE inhibitors (like lisinopril) or angiotensin receptor blockers (like losartan), increases the risk of potassium buildup. Another medication that raises the stakes is spironolactone, a common diuretic that hangs onto potassium. Taking both can push potassium to unsafe levels.
Anyone with diabetes, heart disease, or kidney problems knows how easy it is for potassium to climb too high. The kidneys pull most of the weight in balancing potassium. When they lose strength, waste builds up—and so does potassium. If a doctor prescribes potassium hydrogen malate for kidney stones, but a patient already takes medications that change potassium control in the body, the results can surprise both the patient and the physician.
Some antibiotics, like trimethoprim or sulfamethoxazole, also make it harder for the kidneys to clear potassium. Mixing drugs in this way goes by easily, unless you’re someone who keeps an eye on blood tests. Digoxin, a drug used for heart rhythm problems, plays another dangerous game. High potassium pains the heart, which could send the rhythm out of sync, and stack up complications. Even over-the-counter antacids with aluminum, magnesium, or calcium can shift how potassium gets absorbed. Supplements like potassium hydrogen malate might seem simple, but they show their true colors when other meds enter the picture.
Pharmacists and doctors never stop hammering the point about sharing a current medication list. Even someone taking supplements from the health store should keep the whole care team in the loop. Health outfits like the Mayo Clinic and national drug safety databases warn that not every potassium supplement lists every side effect, and not every label details possible reactions. Regular blood testing turns up small problems before they spiral.
Patient safety happens with clear communication. Anyone starting on potassium hydrogen malate, whether on prescription or from a supplement bottle, should ask about side effects and what warning signs to watch for, like muscle weakness or heart flutter. Keeping a record of changes, updates in health, and new prescriptions makes all the difference. In the world of drug interactions, even a so-called simple mineral deserves respect and careful handling.
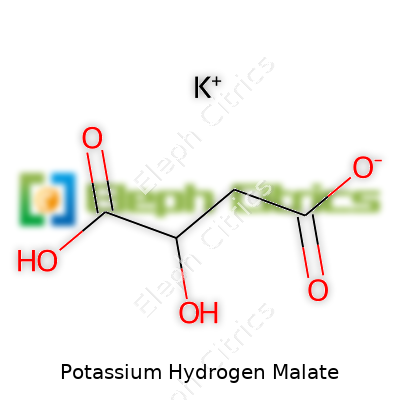
| Names | |
| Preferred IUPAC name | potassium 2-hydroxybutanedioate |
| Other names |
Monopotassium malate Potassium acid malate |
| Pronunciation | /pəˈtæsiəm haɪˈdrɒdʒən ˈmæleɪt/ |
| Identifiers | |
| CAS Number | 617-48-1 |
| Beilstein Reference | 1907932 |
| ChEBI | CHEBI:63315 |
| ChEMBL | CHEMBL1201740 |
| ChemSpider | 63586 |
| DrugBank | DB14557 |
| ECHA InfoCard | ECHA InfoCard: 03-2119726659-31-0000 |
| EC Number | E351 |
| Gmelin Reference | 24779 |
| KEGG | C18718 |
| MeSH | D017690 |
| PubChem CID | 23665718 |
| RTECS number | OJ6300000 |
| UNII | 07L08F125R |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DTXSID0036539 |
| Properties | |
| Chemical formula | KHC4H4O5 |
| Molar mass | 188.18 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.98 g/cm³ |
| Solubility in water | Soluble |
| log P | -2.83 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 5.1 |
| Basicity (pKb) | 11.3 |
| Magnetic susceptibility (χ) | -42.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.473 |
| Dipole moment | 1.65 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 207.7 J⋅mol⁻¹⋅K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1206.5 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1574 kJ·mol⁻¹ |
| Pharmacology | |
| ATC code | A12BA14 |
| Hazards | |
| Main hazards | May cause respiratory, skin, and eye irritation. |
| GHS labelling | GHS labelling of Potassium Hydrogen Malate: "Warning, H319, P264, P280, P305+P351+P338, P337+P313 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | No hazard statements. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | 1-0-1 |
| Lethal dose or concentration | LD50 (oral, rat): >2,000 mg/kg |
| LD50 (median dose) | LD50 (median dose) >2000 mg/kg (rat, oral) |
| NIOSH | Not Listed |
| PEL (Permissible) | PEL (Permissible exposure limit) for Potassium Hydrogen Malate is not specifically established by OSHA. |
| REL (Recommended) | 10 mg/m3 |
| Related compounds | |
| Related compounds |
Malic acid Potassium malate Sodium malate Calcium malate |