Potassium sodium citrate has traveled quite a long road since its initial synthesis. Its family roots in citric acid derivatives run deep within both household and industrial backgrounds. Back in the early to mid-20th century, medical professionals started prescribing citrates to manage urinary tract issues, looking to relieve kidney stones and neutralize uric acid. Chemists figured out they could tweak citrate’s formula by introducing sodium and potassium, changing both its solubility and physiological impact. Over decades, this combination found its way into not only healthcare, but also food preservation and specialized industrial uses, building a record of usefulness supported by growing research and clinical case studies.
Potassium sodium citrate sets itself apart from single-component citrates. It appears as a fine, granular powder or crystalline salt, offering more balanced electrolyte replenishment than ingredients containing only sodium or potassium. Its balanced ratio creates a buffer that helps regulate pH both in living systems and processed foods. You might spot it in oral medications to prevent the crystallization of certain minerals in the kidneys, especially for people with a tendency toward recurrent stones. Packaged for both pharmaceutical labs and industrial bulk supply, labels typically give both the composite and elemental concentrations, reflecting varying needs across applications.
Up close, potassium sodium citrate looks like a white to colorless crystalline powder with a neutral taste and an ability to dissolve easily in water. Melting occurs without decomposition, and the salt holds steady under normal conditions, resisting clumping as moisture stays in check. Chemists measure its molecular weight at around 258 g/mol, creating a handy benchmark for precise formulation work in labs. The pH in solution lands about 8.5, giving it reliable alkalizing properties that draw attention in food tech and medicine alike. No strong odor comes off it, and it remains chemically stable when exposed to air, meaning storerooms stay free of sour smells or hard-to-clean residues.
Manufacturers detail content ratios, moisture limits, and particle size distribution on certificates of analysis. Any supplier worth their salt spells out heavy metal content, residual solvent levels, and microbial counts—nobody wants a contaminant sneaking its way into a clinical batch or commercial food process. Labels follow regulatory trends, not just pharmacopeial monographs. End users check for clear batch numbers, expiration dates, and recommended handling temperatures. Medical settings often demand sterile packaging and dosing instructions to match strict regulatory guidelines for active pharmaceutical ingredients, keeping traceability and compliance at the forefront.
Production starts with citric acid, typically from the fermentation of carbohydrates by Aspergillus niger fungi. Neutralizing this acid with a blend of potassium carbonate and sodium carbonate generates the mixed citrate salt. Chemists control the reaction’s temperature, solution pH, and mixing rate to achieve a consistent final product. They filter and wash the crystallized solid to remove unreacted raw materials. After drying, a final screening step ensures the correct granule size. Some producers opt for spray drying to create faster-dissolving powders for pharmaceutical blends, balancing production scale with purity and functionality.
Potassium sodium citrate acts as a buffer in solution, resisting changes in acidity or alkalinity after the addition of a strong acid or base. This salt can chelate certain metal ions, making it useful in situations where calcium or magnesium buildup causes trouble—say, detergents or medical infusions. Researchers tinker with the salt ratio or pair it with other organic acids to shift solubility or tailor its taste profile. Stability testing under accelerated conditions checks how changes in storage temperature and humidity affect the powder’s properties. Some chemical modifications create derivatives for more niche uses, such as time-release oral medications or injectable electrolyte formulations.
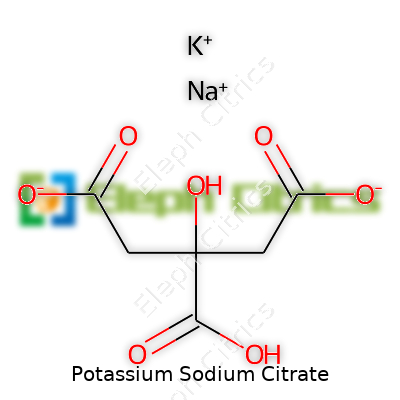
Other names pop up in research articles and on ingredient lists: tripotassium sodium citrate, TPSC, and potassium sodium 2-hydroxypropane-1,2,3-tricarboxylate. Global suppliers add their own spin, calling it by trademarked labels in health products or food processing aids. The mixture’s consistent formula—C6H5KNaO7—anchors it in regulatory filings, even if trade dress varies across brands and countries.
Safe handling starts with clean practices. Plant workers use dust masks and gloves to prevent irritation during transfer and mixing. Storage involves sealed containers away from high humidity, and spill clean-up protocols minimize any slipping hazard or accidental contamination. Production areas stay free from incompatible chemicals, especially acids or oxidizers that could start unwanted reactions. Regulatory agencies like the FDA and EMA look for proof of GMP compliance, certificate records, and thorough hazard communication measures. Pharmacies repackage for patient use using calibrated tools to avoid dosing errors, and shipping follows UN recommendations for chemical safety.
The most common use remains in medical prevention or treatment of kidney stones and certain types of metabolic acidosis. Doctors prescribe it for patients at risk for uric acid or cystine stones, as its buffering action helps maintain urine at a less acidic pH. In the food industry, potassium sodium citrate finds its way into processed cheese, soft drinks, and gel-based candies to regulate acidity and prevent unwanted crystallization. Industrial users employ it in cleaning solutions and water softeners, where its chelating power helps manage hard water minerals that foul up machinery. Sports drinks sometimes feature it for electrolyte balance, although consumer demand for sodium-free formulations shapes its use.
Chemists and clinicians collaborate to explore new uses and delivery forms. Recent studies look at how potassium sodium citrate might improve outcomes for chronic kidney disease patients undergoing dialysis. Food scientists examine whether it can serve as a cleaner-label acid regulator, free from artificial colors or synthetic preservatives. Researchers map out how this salt interacts with other food components—proteins, phosphates, and colorants—to predict shelf stability or flavor changes. On the industrial side, experiments test modified blends in detergents and water treatment schemes, aiming for lower cost and better mineral control without ramping up chemical waste.
Toxicology reviews check both acute and chronic effects. Dose limits come from animal studies and patient monitoring, establishing upper safety boundaries for daily use. Gastrointestinal discomfort, especially at high doses, stands out as a primary side effect—cramping, diarrhea, or nausea pop up in reports. Enough potassium can pose a risk for people with kidney impairment; some studies link excessive intake to dangerous shifts in heart rhythm. Regulatory agencies require rigorous batch testing to catch heavy metal or microbial contamination, with ongoing quality checks following the product from plant to pharmacy or warehouse shelf. Environmental toxicologists look at wastewater handling, ensuring that disposal doesn’t shift local ecosystem potassium levels.
Emerging trends point toward greater customization in pharmaceutical blends, with potassium sodium citrate forming part of wider combination therapies for complex metabolic diseases. Food tech start-ups explore more plant-based fermentation sources, aiming to meet clean-label requirements and sustainable sourcing goals. Industry voices call for greener production pathways, shrinking water and energy footprints while maintaining purity. Regulatory shifts may tighten labeling and traceability rules, placing fresh demand on suppliers for absolute transparency. As digital tools take hold, laboratories track batch performance in real-time, improving consistency and safety for everyone down the line—from factory staff to end users picking up a prescription or packet at the store.
Potassium sodium citrate turns up in some unexpected places. A doctor introduced me to it during my own experience with kidney stones. Many people don’t know this ingredient helps the body manage acids and minerals in a way that’s hard to ignore if you value your kidneys or bladder. It acts by making urine less acidic, which reduces the risk of certain minerals building up and forming stones. Anyone who’s struggled with that pain knows what a difference even a slight reduction in risk can make.
People with gout, another deeply uncomfortable diagnosis, sometimes hear about potassium sodium citrate because it can break down uric acid crystals. Fewer crystals mean less swelling, and life starts to feel a bit more normal. That’s a real win for quality of life. It also shows up in managing certain types of metabolic acidosis—where the body turns too acidic usually because the kidneys struggle to filter waste. With the kidneys under pressure, doctors use potassium sodium citrate to help restore a healthier balance. That’s backed up by studies in nephrology journals, not just patient stories.
Beyond medicine, food scientists and beverage companies use potassium sodium citrate to tweak flavors and control acidity in products like soft drinks or processed cheese. Walking through a grocery aisle, you might not spot it on every label, but it plays a role in making some foods more stable and safer to eat. It helps preserve color and texture, making the eating experience a little better without fanfare. From personal study and plenty of snack labels, I’ve noticed that potassium sodium citrate quietly supports freshness in more packaged foods than most realize.
No solution feels complete without looking at problems. High doses of potassium sodium citrate can affect people with weak kidneys or with certain heart problems. That’s something I learned after seeing a family member with kidney disease have their medications adjusted. Doctors pay careful attention because an electrolyte shift can set off more trouble than it solves—irregular heartbeat or muscle weakness, for example. My research into the FDA guidance tells me medical supervision is a must, not a suggestion. That lesson stuck with me, and I make a point to ask questions now at every doctor’s visit.
Potassium sodium citrate offers relief for some and helps industry maintain food quality. But informed use makes the biggest difference. Easy-to-read labels on food products, or clearer guidelines in pharmacies, could keep people safer. Making sure people know when to avoid it—like certain kidney or heart conditions—would cut down the number of folks who run into trouble. Training nurses and pharmacists to ask the right questions turns safe use from a guess to a sure thing. I learned first-hand that better conversation and awareness go further than simply handing over a prescription. By focusing on practical steps and honest discussion, we can unlock more benefits from this overlooked compound while keeping risks in check.
Potassium sodium citrate doesn’t come up much at a backyard barbecue. Most neighbors could list a handful of over-the-counter meds, but say “potassium sodium citrate” and you’re likely to get blank stares. For those with kidney stones or certain urinary tract troubles, this compound is more than a mouthful. It matters. Kidneys work best when acids and salts stay in balance, and this medicine helps shift that balance where it needs to go. Doctors hand out potassium sodium citrate to manage acid levels, protect against stones, and sometimes to relieve symptoms from chronic urinary tract conditions.
A few years back, I watched a close friend grapple with kidney stones. The pain was like a freight train. His doctor prescribed potassium sodium citrate. I saw my friend wrestle with the instructions. At first glance, the label seemed straightforward. Pour the syrup or dissolve the tablet, drink with water. Simple, right? Not quite. The details matter. Skipping water can burn the stomach. Taking it without food causes nausea. My friend learned fast: always mix tablets or syrup with at least a half-glass of water and pair the dose with meals. This little ritual spared him a lot of discomfort.
Doctors recommend timing doses with meals for a reason. Potassium and sodium, both electrolytes, help nerves and muscles, but the gut often protests against direct hits of strong salts. Eating first gives the stomach a buffer. My friend once skipped food, took the medicine straight, and spent an hour hunched over in pain. That day became a lesson in following instructions.
The biggest mix-up I’ve seen: people forget to hydrate. Without enough water, the solution can feel harsh going down, and kidneys get stressed. I know someone who thought the water part was just a suggestion. His doctor made it clear: the water protects the stomach and helps the kidneys flush out the excess salt efficiently. Forgetting doesn’t just rough up the digestive tract—it can strain the very organs the medicine aims to protect.
Following the prescribed amount matters more than many realize. Doubling up to “catch up” after a missed dose can send potassium levels soaring, which sends the heart into dangerous rhythm. Every prescription I’ve seen came with clear directions—stick to the routine, don’t double up, call the doctor if something feels off or you forget a dose. Pharmacists and doctors both hammer home the message: don’t reinvent the dosing schedule on your own.
The most effective fix starts with patient-doctor talk. Ask about the right foods, best times, and ways to avoid upset stomach or cramps. A little curiosity and clear communication pay off. Reading the label out loud, texting reminders for each dose, and planning meals around the medicine prevent most mistakes. Pharmacists, nurses, even friends who’ve walked this road before—these folks carry tips you won’t find on the label. Simple tricks, like keeping the bottle near the breakfast table and setting a phone alarm for the evening dose, can sustain the habit for weeks or months.
Health advice turns real once you live with chronic conditions. The biggest help: connect dosage to real routines—breakfast, dinner, even a daily TV show. Personal strategies take you further than any handout because each life looks different. Potassium sodium citrate has a job to do, and with care, it gets the job done without derailing normal days.
I remember sitting in a clinic with a friend struggling with kidney stones. The doctor prescribed potassium sodium citrate, saying it could help keep stones from coming back. Just like that, we all nodded and trusted the process. A few weeks in, though, my friend joked he had turned into a “side effect machine.” Nausea that wouldn’t quit, this weird metallic taste in his mouth—suddenly, everyday life felt a little more complicated.
People taking potassium sodium citrate often report nausea, vomiting, stomach cramps, or even diarrhea. It’s easy to shrug it off as “tummy trouble,” but these side effects can send folks running for the bathroom or feeling queasy all day. For some people, these issues fade as the body gets used to the medicine, but others never really adjust. I’ve seen people stop taking the medication entirely, putting their treatment at risk. Doctors usually recommend taking it after meals or with plenty of water, hoping to cushion the blow to the digestive system.
This medicine can tip the balance of sodium and potassium in the blood. Most folks won’t notice it unless they get blood tests, but anyone with kidney disease, heart issues, or high blood pressure could be especially vulnerable. Too much potassium (hyperkalemia) causes symptoms like muscle weakness or, in serious cases, a heartbeat that just doesn’t sync right. Too much sodium (hypernatremia) sometimes leads to confusion, swelling, or headaches. People who care about their health learn to pay attention—not in an anxious way, just aware and honest when describing any new symptoms.
One large review in the American Journal of Kidney Diseases found that up to one in five patients experienced gastrointestinal upset. Fewer people see dangerous blood imbalances, but it happens—especially in older adults or folks with other chronic conditions. There’s a reason doctors check electrolytes with those regular blood draws. The U.S. National Library of Medicine lists both mild symptoms (stomach upset, headaches) and serious red flags (trouble breathing, arrhythmias, muscle paralysis) in their official safety data.
Doctors keep a close eye on people using potassium sodium citrate. They may recommend starting with a low dose and working up, spaced through the day. Plenty of fluids make a difference—both for the stomach and the kidneys. I’ve seen doctors teach patients what to watch for, like unexplained fatigue or a heartbeat that feels “off.” Sometimes a person just needs a different medicine or more frequent lab checks.
No medicine comes without tradeoffs. Open conversations with healthcare teams, honest reporting of symptoms, and a willingness to ask questions all help people stay safer, even when side effects make things rough. Potassium sodium citrate does help many folks manage kidney stones and certain metabolic issues, but the side effects remind us that every pill has power. There’s no shame in asking for help, getting a blood test, or saying out loud, “Something doesn’t feel right.” That’s how real life and good medicine meet.
Many women juggle nausea, indigestion, and acid reflux through their pregnancies. Potassium sodium citrate lands on the list of medicines that hit the pharmacy shelves with claims of relief. It’s a salt that helps neutralize stomach acid and supports the body’s acid-base balance. But pregnancy and breastfeeding bring a different set of rules. Every medication or supplement becomes something to second-guess, especially with stories running rampant online about surprise side effects.
Doctors sometimes use potassium sodium citrate to help treat kidney stones or manage too much acidity. The compound has a long track record, and its electrolytes—the potassium and sodium—support body function when prescribed for the right reason. For example, those with a history of kidney problems might need it after careful blood tests, since imbalance in body salts can bring real trouble.
The real question pops up for expecting or breastfeeding mothers: Where’s the evidence? Most medical guidelines lean on caution, mainly because big studies about potassium sodium citrate and pregnancy just don’t exist. Research stays scarce, and drug makers provide very little information. So decisions often rest on in-the-room conversations between patients and health care professionals, weighing the benefits of easing symptoms against the unknowns.
One overlooked issue is that potassium and sodium both play huge roles in blood pressure. Many women already face shifts in blood pressure during pregnancy. Adding an extra source—especially when not under direct doctor guidance—can risk tipping that delicate balance further. Getting too much potassium, for example, could affect how the heart works or even harm kidney function. An overload of sodium could feed into swelling or blood pressure concerns already present in many pregnancies.
Breastfeeding mothers face similar issues. What passes into breast milk could end up in the baby’s system, and medical literature gives few solid facts about potassium sodium citrate’s safety for infants. There’s no clear picture of the risk, but with so much still unknown, that keeps the advice conservative.
A prescription from a doctor usually means a careful review of your overall health, kidney and heart status, and current medications. This isn’t an area for self-diagnosing or grabbing over-the-counter remedies on a whim. My own family dealt with early labor scares because of a supplement taken without medical input—it was an eye-opener about how quickly “harmless” can turn into risky. There’s wisdom in leaning on the people who study this for a living.
If heartburn or digestive issues drive the urge to reach for potassium sodium citrate, talking to a doctor can uncover other options. Simple steps like tweaking meals, staying upright after eating, and controlling portion size often help reduce symptoms. Some women benefit from antacids considered safer by pregnancy experts. Doctors might recommend regular blood tests for those who truly need potassium sodium citrate or similar treatments, just to watch for changes in body salt levels.
At the end of the day, the stakes shift during pregnancy and breastfeeding. Playing it safe and pushing for answers brings more peace of mind than any guesswork. Health care teams make an important partner for sorting through noise and making well-grounded choices, especially for mothers who carry not just their own hopes, but also those of a new life.
Potassium sodium citrate gets plenty of use in managing kidney stones and certain urinary issues. It's popular in both hospital and outpatient settings. Most people see it as a routine over-the-counter solution, especially for those battling conditions that really test patience, like recurring kidney stones or metabolic acidosis. The straightforward nature of this medication sometimes makes patients forget how it can mix with other drugs.
Anyone with a cabinet full of prescription bottles should stop and think before starting potassium sodium citrate. Even though it sounds simple, this medication changes the body’s chemistry. It boosts the body’s levels of potassium and sodium, and nudges the blood towards a less acidic state. That matters for people taking drugs such as ACE inhibitors, angiotensin receptor blockers (ARBs), and potassium-sparing diuretics like spironolactone or amiloride. With those medicines, potassium levels already trend higher. Stack potassium sodium citrate onto that, and potassium could push dangerously high, leading to confusion, weakness, or even heart rhythm problems.
Other medications can complicate things, too. People taking digoxin—often prescribed for heart conditions—should stay alert. Shifts in potassium and sodium change how digoxin acts inside the body. Too much or too little of either ion could tip the scales toward serious side effects. Diuretics present another concern. Loop and thiazide diuretics may lower potassium, which might seem like a good reason to add potassium sodium citrate. Still, those shifts can be unpredictable without frequent blood tests.
Beyond specific drug names, several chronic conditions demand extra caution. Those with chronic kidney disease already have a rough time clearing potassium. Adding more without tracking blood work can quietly lead to hyperkalemia. Similar risks show up for people living with Addison’s disease or other hormonal disorders, where sodium and potassium balance can break down quickly. Healthcare providers rely on up-to-date labs before green-lighting these kinds of combinations, and rightfully so.
Plenty of people think of vitamins and supplements as “natural” and harmless, yet even common supplements can interact with potassium sodium citrate. Black licorice, salt substitutes, or herbal cleanses sometimes pump up potassium or drain sodium without warning. These details may seem small until they snowball into larger problems. It pays off to keep an open dialogue with pharmacists and doctors. Getting regular blood tests gives a real-time view of how all these chemicals are playing together inside the body.
Sensible medication management starts with honest self-reporting. Many times while interviewing patients, it surprises me how often key information only comes out after a casual conversation rather than a painstaking checklist. Providing a thorough medication list—including supplements and vitamin drinks—to every healthcare provider makes it easier to spot early warnings. Bringing up side effects such as muscle weakness, heart palpitations, or digestive changes leads to faster responses.
Electronic health records also cut down on errors. Pharmacy alerts sometimes catch things missed during a busy clinic day. Anyone juggling multiple prescriptions should look for one pharmacy that can track all their refills. Even a ten-minute medication review once or twice a year makes a difference.
Potassium sodium citrate can offer real relief, but that peace of mind comes from understanding the risks. Open communication, frequent lab checks, and informed choices transform what could be a dangerous guessing game into safe, effective treatment.
| Names | |
| Preferred IUPAC name | potassium trisodium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Trisodium citrate and tripotassium citrate mixture Potassium trisodium citrate Citrate mixture Citrosodine |
| Pronunciation | /pəˈtæsiəm ˈsoʊdiəm ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 144-33-2 |
| Beilstein Reference | 3921146 |
| ChEBI | CHEBI:133325 |
| ChEMBL | CHEMBL1201132 |
| ChemSpider | 22316478 |
| DrugBank | DB01326 |
| ECHA InfoCard | 100.233.665 |
| EC Number | E337 |
| Gmelin Reference | 81137 |
| KEGG | C14224 |
| MeSH | D018456 |
| PubChem CID | 23682535 |
| RTECS number | WM8856000 |
| UNII | 1U8H079U6S |
| UN number | UN2818 |
| CompTox Dashboard (EPA) | DTXSID2078936 |
| Properties | |
| Chemical formula | KNaC6H5O7 |
| Molar mass | 324.36 g/mol |
| Appearance | White or almost white, crystalline powder or colourless crystals |
| Odor | Odorless |
| Density | DENSITY: 1.98 g/cm³ |
| Solubility in water | soluble |
| log P | -4.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 5.7 |
| Basicity (pKb) | pKb ≈ 3.6 |
| Magnetic susceptibility (χ) | −54.0·10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.451 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 417.3 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -230.0 kJ/mol |
| Pharmacology | |
| ATC code | A12BA03 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Do not ingest. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Lethal dose or concentration | LD50 Oral (Rat) >2000 mg/kg |
| LD50 (median dose) | LD50 (median dose) > 2,000 mg/kg (rat, oral) |
| PEL (Permissible) | Not established |
| REL (Recommended) | 3-6 g daily |
| Related compounds | |
| Related compounds |
Sodium citrate Potassium citrate Citric acid |