Propyl lactate emerged long before the world recognized its potential. Chemists in the late 19th century tinkered with lactic acid, always on the lookout for esters that could offer better solvent properties and easier handling. Natural sources such as fermentation broths and sour milk gave rise to lactic acid, and soon, clever synthesis methods unlocked a family of lactate esters, with propyl lactate standing out for its distinct solvent profile. Industrial interest sprouted as manufacturers needed safer, biodegradable alternatives to harsh solvents used in paints, inks, and flavorings. Over the decades, improvements in purification, green chemistry, and fermentation have quietly made propyl lactate a behind-the-scenes hero in labs and factories.
Propyl lactate typically turns up as a clear, colorless liquid, easy to pour and store. It rarely gets the spotlight outside of technical circles, yet it underpins efforts to push away from petroleum-based solvents. Its low volatility pleases those trying to rein in workplace hazards. Companies that care about reducing their carbon footprint turn to propyl lactate not just because of its biodegradable backbone but also for its bite-sized odor profile that doesn't choke production lines.
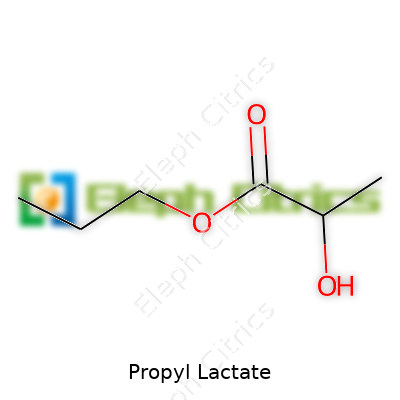
At room temperature, propyl lactate stays as a mobile liquid, thanks to its moderate molecular weight and low melting point. Its chemical formula—C6H12O3—tells the real story. That little ester group locks in stability, even as it brings just enough polarity to dissolve stubborn resins and polymers. The boiling point sits at about 181°C, high enough to make it less prone to spiraling off during processing. With a flash point comfortably above 75°C, facilities chasing safer working conditions find it far less risky than lighter, more flammable solvents. Solubility matters on factory floors, and propyl lactate handles mixing chores well. It works with water to a degree but truly excels at blending in with oils and other organics.
Every drum or bottle of propyl lactate needs a clear label. Companies put down purity standards, usually topping 98%, along with residual acid and moisture specs, for anyone watching product consistency. Batch-to-batch color can vary slightly, but that rarely disrupts downstream use. Key identifiers like CAS number (623-17-6) and EC number (210-785-4) trace each shipment right back to its source. Technical sheets typically list refractive index and specific gravity, giving end users a glance at the crucial details for critical processes. Industry certifications such as ISO 9001 or REACH compliance hang heavy in purchasing decisions, often making or breaking adoption in regulated sectors.
Production lines for propyl lactate usually start with lactic acid, itself brewed from renewable carbohydrate sources using well-tuned bacteria. The acid meets n-propanol under the watchful eye of a strong acid catalyst, most often sulfuric acid, in a classic esterification reaction. Producers strip away excess alcohol and water using vacuum distillation, squeezing the product to high purity. Personal experience in a pilot plant highlighted how tight control on reaction times and temperatures keeps side products down, saving headaches further down the line. Industry has started to pivot toward solid acid catalysts and continuous flow systems, cutting waste and making clean-up easier. Greater use of sustainable feedstocks moves the process toward circularity as markets demand stronger green credentials.
Propyl lactate’s chemical backbone looks deceptively simple, but subtle tweaks can unlock a range of properties. Nucleophilic attack on its ester group, for example, breaks the molecule back to lactic acid and n-propanol under strong base—useful for controlled hydrolysis. It reacts smoothly in transesterification steps, letting chemists swap out the propyl for other groups as needed. Reaction with mild oxidizers, though rare in product use, can stress-test container compatibility over long-term storage. In the lab, propyl lactate sometimes takes part in polymerization reactions or acts as a solvent for biocatalysts, showing surprising versatility outside the usual paint and flavor circuits.
The world knows propyl lactate by a handful of names: 1-propyl lactate, n-propyl 2-hydroxypropanoate, and 2-hydroxypropanoic acid propyl ester. Older literature might dub it “lactic acid, propyl ester.” Marketed products carry branding tied to their end-use sectors, with paint and coating blends touting “eco-friendly ester solutions” and flavor houses labeling it under food-grade descriptors. Chemists picking through catalogs scan for all these phrases, lest they miss a crucial supplier.
Handling chemicals safely takes more than a cursory glance at a safety icon. Propyl lactate, while mild compared to industrial solvents like toluene or methyl ethyl ketone, still puts pressure on safety teams to educate workers. Liquid or vapor can irritate eyes and skin during transfer, often requiring gloves, goggles, and efficient ventilation. OSHA-compliant Material Safety Data Sheets detail recommended exposure limits and first aid guidance. In warehouses, barrels sit capped, always labeled, away from oxidizers and strong bases. Companies committed to environmental stewardship set up spill containment and waste-water treatment, meeting both local and global regulations. A personal look at a large coating plant revealed that investing in modern fume extraction made a world of difference for employee comfort and retention.
Propyl lactate quietly fuels progress across industries. Paint shops favor it for fast, even drying of coatings, all while children and pets play nearby—an edge over older solvents that brought health worries. Print shops benefit from propyl lactate’s gentle odor, even as staff turn out high-volume jobs in tight quarters. The food sector leans on it as a flavoring additive or extractant, often under close scrutiny from regulatory bodies like the FDA, which generally regard it as safe in small quantities. Specialty plastics and adhesives companies now fold propyl lactate into greener product lines, meeting consumers who look for “low VOC” or “natural solvent” on packaging. In the world of pharmaceuticals, it sometimes features in topical creams, its low toxicity opening doors that petroleum derivatives can’t.
Researchers target propyl lactate for its role in creating low-impact formulations, especially as policy shifts and consumer attitudes bring green chemistry to the front page. Studies explore catalysts that shorten reaction times or pump up yields, making production nimbler and cheaper. Cutting-edge labs in Europe and North America chase ways to recycle by-products or switch entirely to enzymatic production, promising smaller carbon footprints. Startups experiment with blends of propyl lactate and other esters to tackle ever-tougher paints and plastics, trying to leapfrog the performance of tradition. In the flavor and fragrance world, research dives into optimizing taste notes while side-stepping off flavors, all in pursuit of healthier, more appealing food additives.
Propyl lactate draws attention for its relatively low toxicity, one reason it loosens previous reliance on nastier chemicals. Toxicological studies, including work supported by agencies such as the European Food Safety Authority, indicate that propyl lactate metabolizes to lactic acid and propanol—both of which the human body handles naturally, barring excessive doses. Oral, dermal, or inhalation exposure tests show minimal health impacts at typical occupational or consumer levels. Chronic toxicity concerns drop further due to its ready biodegradability and low likelihood for bioaccumulation. Even so, regulatory updates caution against careless use around food preparation or in children’s products, since manufacturing impurities or improper handling could shift the risk profile. Personal observation from work with regulatory filings confirms the importance of routine purity checks and transparent sourcing.
Strong momentum builds behind propyl lactate as pressure for eco-friendly solvents intensifies. Sectors such as automotive coatings and electronics cleaning start to peer beyond the old guard of aggressive, slow-to-breakdown solvents, seeking alternatives that please customers and regulators. Upcoming innovations likely focus on engineering bespoke blends for tight specs, tuning volatility and evaporation rates without trading away safety. Biotechnology firms look at feeding waste lactic acid streams right back into production, supporting closed-loop supply chains that drive cost and environmental benefits. The days of hiding chemistry behind opaque labels give way to campaigns that transparently tout sourcing, safety, and end-of-life profiles. With steady research and open communication among regulators, manufacturers, and users, propyl lactate stands primed to factor heavily into how companies tackle sustainability and worker health for decades to come.
Propyl lactate does not pop up in most conversations, but that does not make it any less important in everyday products. Derived from lactic acid and propanol, this compound takes the form of a clear, oily liquid. You can find it by a few different chemical names, though most people run into it on an ingredient list rather than a chemistry book.
As someone who spends the weekend scrubbing the house or fixing up workshop projects, I have run into propyl lactate in some unexpected spots. This ingredient slips quietly into cleaning products, inks, coatings, and sometimes even food flavors. Manufacturers like using it because it helps dissolve grease and sticky messes without the harsh smell or skin irritation that comes with stronger chemicals.
A bottle of graffiti remover or industrial degreaser often lists propyl lactate. It breaks down oily stains and helps water-based cleaners work better. Printing shops use it in ink formulas for smoother, more vivid results. Paint shops also mix it into coatings to keep paint layers level and glossy, with fewer bubbles or streaks.
Propyl lactate also plays a part in making products less harsh on the nose or skin. Some solvents burn your lungs or hands, but this ingredient offers a gentler alternative. I notice fewer headaches cleaning the garage with products that swap out traditional solvents for this kind of compound.
You might taste propyl lactate before noticing it on a label. The food industry leans on it for fruity, slightly sweet flavors, especially in baked goods and candies. It’s labeled as generally recognized as safe (GRAS) for certain uses by the FDA, which reassures parents and cooks. That matters for anyone trying to steer clear of harsh additives in their family’s meals.
Perfume and personal care products have a softer, more pleasant scent thanks to this chemical. Its mild, natural-smelling fragrance blends well instead of overpowering. Small things like this can make a difference for people sensitive to artificial scents.
Even safe ingredients like propyl lactate deserve attention. Research shows it breaks down easily in the environment and does not usually cause irritation. Still, long-term exposure to any chemical can cause surprise issues. I always try to keep gloves handy and work in a ventilated space, especially during big cleaning jobs.
For folks with chemical sensitivities, the search for safer alternatives never ends. Some companies are moving toward even milder plant-based solvents as technology and consumer demand shift. Watching how regulations develop can help everyone keep their homes and businesses a little safer.
Even if you do not work in a laboratory, knowing about ingredients like propyl lactate lets you make smarter choices. Whether choosing paint for a nursery, a cleaner for the kitchen, or dessert for a birthday party, you gain control by understanding what goes into those products. Trust grows when people know what’s in the bottle or on the plate.
Reading past the marketing labels matters. Propyl lactate might not be a household word, but learning where it shows up puts power back in your hands. And as more folks ask questions about what goes into their homes and bodies, manufacturers keep listening and improving what they sell.
Propyl lactate comes from lactic acid and propanol, making it an ester with a light, pleasant odor and the ability to dissolve in fats and oils. It’s been used for years in a range of products, especially as a flavoring in foods and sometimes as a solvent in cosmetics and cleaning agents. Anyone who enjoys baked goods, candies, or ice cream likely tasted small amounts of it without even knowing.
Big food safety organizations have paid attention to propyl lactate. In the United States, the Food and Drug Administration (FDA) includes it on the list of substances “Generally Recognized as Safe” (GRAS) when it’s used as a flavoring. In Europe, the European Food Safety Authority (EFSA) evaluated it and gave it the thumbs up for specific uses in foods. These agencies don’t just give rubber stamps; it takes lab tests, independent studies, and multi-year reviews to reach a conclusion about a substance’s safety. In my time exploring food safety cases, extensive data always backs up a GRAS designation.
Once inside, the body breaks propyl lactate down into lactic acid and propanol. Both of these are handled pretty easily by healthy adults. Lactic acid gets metabolized for energy, and propanol gets processed in the liver. Most people would struggle to avoid lactic acid—our own muscles make it during exercise. Propyl lactate just brings it in a different package. The quantities used in food are very small and rarely cause side effects under normal circumstances.
Questions about possible health risks often focus on people with allergies or unique metabolic disorders. Some may be sensitive to certain additives or have underlying conditions impacting how the body breaks down esters or alcohols. Very high doses could bring discomfort, especially stomach irritation, but such numbers far exceed normal exposure. Occupational exposure matters for people working in factories, not everyday shoppers grabbing a snack.
The Environmental Working Group (EWG) rates propyl lactate as “low hazard” for use in cosmetics, basing this on a lack of evidence for significant toxicity or allergy risk in typical conditions. Scientific literature doesn’t show convincing ties to cancer, hormone disruption, or birth defects.
Even with a solid safety record, it still makes sense for regulators to keep tabs on new research. Food manufacturers and cosmetic brands should label ingredients accurately and keep quantities well below safety thresholds. Anyone with a history of food sensitivities benefits from paying attention to ingredient lists and reaching out to healthcare providers for advice.
There’s always value in supporting transparent science and consumer education. People have a right to know what’s in their food and personal products, and they deserve honest communication from both businesses and public health groups. If new evidence comes up that challenges old assumptions about propyl lactate, food safety agencies around the world have systems to respond quickly.
Propyl lactate pops up as a clear liquid, usually with no color. Its aroma reminds me of green apples—fresh and faintly sweet. Walking into a lab where propyl lactate gets used, I’ve always caught that gentle, fruity smell that’s never overpowering, just a little reminder of why this chemical goes into flavors and fragrances. Even folks working with solvents daily will likely recognize propyl lactate by scent alone.
Each molecule has three carbon atoms attached to the lactate core, which is what science calls an ester formed from the reaction between lactic acid and propanol. Propyl lactate’s formula: C6H12O3. Its molecular shape means it dissolves easily in organic liquids such as alcohols or acetone, but spills don’t mix easily with water. The boiling point sits around 185-190°C—heating it up won’t make it jump to gas right away, adding a bit of safety in manufacturing.
Because of its low viscosity, pouring propyl lactate feels a lot like handling vegetable oil. This works out for people measuring and mixing liquids by hand or automation. Its density is slightly less than water, hanging out at about 1.04 g/cm³, so a liter weighs just a bit more than a liter of milk. If it gets on your skin, you’ll notice it feels smooth and dries out quickly, especially in a warm room.
What makes propyl lactate valuable is its stability. Sitting on a shelf, it resists breaking down for a long time, which fits the needs of food and cosmetic makers looking for shelf life. It also isn’t known for being highly flammable, but the flash point still hits 64°C. Anyone pouring, storing, or moving this solvent in large quantities needs decent ventilation and basic fire safety practices.
High purity brings out the best from propyl lactate, especially for uses in flavors and fragrances. Contaminants can change the taste, smell, and even safety. I wouldn’t drink a soda with questionable solvents, and neither would most folks. In my work, keeping solvents clean has always paid off—lower risk of odd scents, no worries about unexpected side effects. Solubility in oils means flavor formulators can deliver the apple note to baked goods or candy without messy separation.
Propyl lactate avoids some pitfalls of older, harsher solvents. For industries chasing safer workplaces and less toxic waste, this property helps it replace solvents known to harm people or pollute water. Regulations push companies to swap out hazardous ingredients, and propyl lactate often lands on lists of “greener” options for paints, nail polish removers, and food flavorings. If spills do happen, cleaning up requires proper gloves and good ventilation. Avoiding contact with eyes or long skin exposure keeps things safe.
With more companies aiming for sustainable production, the search for bio-based and biodegradable solvents keeps rolling. Propyl lactate, made from fermenting renewable sources, checks both boxes. Switching standard solvents for something that leaves less of a mark on the environment just makes sense.
National Center for Biotechnology Information; U.S. National Library of Medicine, PubChem Compound Summary for Propyl lactate. European Food Safety Authority, Scientific Report on Flavouring Group Evaluation. The Merck Index – An Encyclopedia of Chemicals, Drugs, and Biologicals.
I’ve seen the ripple effects that come from handling chemicals the wrong way. Propyl lactate, often used as a solvent in food flavoring and industrial processes, poses certain risks—sometimes overlooked. Accidental spills or inhalation can bring on headaches, dizziness, or even worse issues. Fires can start in storage areas if folks ignore the basics. Not everyone likes the smell or the irritation it causes, either.
Propyl lactate reacts to heat and air a lot faster than water or vinegar. Most people keep it in a cool, well-ventilated room, away from sunlight and ignition sources. I always double-check that the storage area sits far from heavy foot traffic or spots where workers eat and take breaks. Steel shelves help in maintaining stability, but I prefer to use corrosion-resistant containers for storing the liquid. This step cuts down the risk of leaks and reacting with the storage material itself.
Keeping storage drums fully closed also slows evaporation and limits the amount of vapor in the air. Vapors from propyl lactate can build up even with small slip-ups, leading to respiratory problems or fire hazards. Regularly inspecting container seals and labels makes a difference, since labels fade or peel over time. Safety data sheets, required by OSHA, highlight these details too, but nothing beats a daily walkthrough and quick glance at the conditions.
I like to approach chemical handling the way I approach car maintenance: care at every step. Wearing gloves and safety glasses reduces skin and eye irritation—no one gets used to that burn, even after years on the job. Proper ventilation, with exhaust hoods or open windows if indoors, keeps vapors from reaching dangerous levels. Folks who work with propyl lactate a lot should learn how it smells. Sudden changes mean something’s up.
No food, drinks, or loose items around while using or transferring the liquid, as these can collect the chemical and raise the risk of accidental ingestion. Some might get relaxed around small volumes. From what I’ve seen, it’s better to treat even small bottles with the same caution as a fifty-gallon drum.
Spills happen, often at the worst times. I keep absorbent pads or clay-based spill kits handy. Once, a minor leak went unnoticed under a shelf for a couple of days, leading to ruined floor tiles and a nasty smell that stuck around for weeks. Quick cleanup with plenty of fresh air saves headaches and long-term damage. Store used cleanup materials in sealed bags, preferably outside the main storage area, until proper disposal.
No amount of good storage or careful handling fixes ignorance. Training staff on chemical hygiene keeps routines tight and accidents rare. I encourage crew members to question anything that feels off; mistakes drop when everyone looks out for each other. Posting emergency numbers, eye wash stations, and spill plans near the storage area means anyone can respond fast if something goes wrong.
Propyl lactate, like any chemical, calls for respect—not fear. Safe handling and storage preserve health and property, saving everyone from problems down the line.
Propyl lactate pops up in food processing, cosmetics, paints, and even cleaning products. It's considered an ester, coming from lactic acid and propanol. I’ve worked in both industrial kitchens and small-scale labs, so I’ve handled ingredients and solvents like this firsthand. The way chemicals break down after doing their job always affects people and the places where we live.
Many companies say their products break down nicely in the environment, but let’s focus on facts. Propyl lactate doesn’t hang around forever. Microbes in soil and water chew it up, turning it mostly into carbon dioxide and water. Scientific sources like PubChem and environmental reports show that natural bacteria gobble up this chemical pretty quickly — the numbers point to over 60% breakdown in less than four weeks under lab conditions.
In my experience teaching high school students about environmental science, kids are always relieved when a substance doesn’t pollute rivers. Propyl lactate scores well here, since it won’t linger and build up where plants and fish live. The whole process keeps it out of long food chains, unlike some old-school solvents that stick around for decades.
Waterways catch most of the runoff from manufacturing and everyday use. Nobody wants to see factories feed more chemicals into streams after rinsing equipment or washing hands. Research shows propyl lactate dissolves easily, and breaks down under natural sunlight and with the help of native bacteria — both freshwater and saltwater. My own small garden, which feeds on city tap water and compost, reflects this: leafy greens do just fine, and soil tests never reveal traces of these biodegradable esters. Direct spills and large releases could cause temporary problems, but nothing sticks as a long-term contaminant.
Air pollution is another question. Propyl lactate isn’t volatile like acetone, so it doesn’t float off into the air easily. That means you don’t breathe in big doses, and city air quality doesn’t dip just because workers use it in a factory or bakery. Most safety data shows mild annoyance to skin or eyes, and just plain old vinegar-like smells in poorly ventilated areas. Always wear gloves and goggles, but the risks drop compared to alternatives loaded with harsh petroleum.
No chemical comes without its shadows. Mass production of lactic acid, usually from corn or plant sugars, burns up energy. If a country grows corn using heavy pesticides or fertilizers, propyl lactate can’t claim full “green” status. There’s also the cost: companies argue over price points, and some cut corners. Factories overseas may lack stricter rules, raising questions about fairness and environmental oversight.
The solution sits with transparency. Manufacturers who source raw materials responsibly, track energy use, and share their environmental impact win public trust. Clean labels, plus third-party testing, help consumers like me pick products with a lighter environmental touch. Local governments and businesses can push for open reports about water, air, and soil testing near production sites.
Propyl lactate’s story isn’t perfect, but tests and real-world results support the claim: it breaks down fast, harms little, and doesn’t hang over ecosystems like older ingredients do. The real challenge lies in pushing producers to prove their supply chains don’t hide dirty secrets.
| Names | |
| Preferred IUPAC name | Propyl 2-hydroxypropanoate |
| Other names |
Propyl 2-hydroxypropanoate Propyl 2-hydroxypropionate Lactic acid propyl ester Propyl lactat Propyl ester of lactic acid |
| Pronunciation | /ˈprəʊpɪl ˈlækteɪt/ |
| Identifiers | |
| CAS Number | 597-82-0 |
| Beilstein Reference | 1818732 |
| ChEBI | CHEBI:81829 |
| ChEMBL | CHEMBL135088 |
| ChemSpider | 10643 |
| DrugBank | DB11265 |
| ECHA InfoCard | 03b225fab452-4440-92c5-14674f4fd32b |
| EC Number | 211-915-7 |
| Gmelin Reference | 7417 |
| KEGG | C18583 |
| MeSH | Dodecanol: C10H21CHOHCOOC3H7 |
| PubChem CID | 8023 |
| RTECS number | OD9625000 |
| UNII | 1A3O67436H |
| UN number | UN3272 |
| Properties | |
| Chemical formula | C6H12O3 |
| Molar mass | 118.17 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | sweet;rum-like |
| Density | 0.997 g/mL at 25 °C |
| Solubility in water | Miscible |
| log P | 0.51 |
| Vapor pressure | 0.27 mmHg (20 °C) |
| Acidity (pKa) | 16.0 |
| Basicity (pKb) | pKb ≈ 15.3 |
| Magnetic susceptibility (χ) | -7.44×10^-6 |
| Refractive index (nD) | 1.419 |
| Viscosity | 2.4 mPa·s (20 °C) |
| Dipole moment | 3.25 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 353.1 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -589.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2161.0 kJ/mol |
| Pharmacology | |
| ATC code | A16AX10 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | H315, H318, H335 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P305+P351+P338, P370+P378 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Flash point | 120 °C |
| Autoignition temperature | 260 °C |
| Explosive limits | 2.5–17.5% |
| Lethal dose or concentration | LD50 Oral Rat 6730 mg/kg |
| LD50 (median dose) | LD50 (median dose): 6,760 mg/kg (oral, rat) |
| NIOSH | STY |
| PEL (Permissible) | PEL: Not established |
| REL (Recommended) | 0.5 mg/L |
| IDLH (Immediate danger) | 250 ppm |
| Related compounds | |
| Related compounds |
Methyl lactate Ethyl lactate Butyl lactate Isopropyl lactate Lactic acid Propyl acetate |