Tracing the roots of silver lactate takes us to the crossroads of organic chemistry and metal compounds, where inventive chemists observed how silver ions interact with organic acids like lactic acid. During the nineteenth century, European researchers documented numerous silver-organic salts, recognizing their potential for both medical and industrial use. Back then, medicine leaned heavily into silver’s antimicrobial charm, but these discoveries broadened the range of silver chemistry. Preparation techniques improved, shifting from laborious precipitation with crude lactic acid solutions toward methods with cleaner, more pure reactants and refined crystallization. The gradual adoption of analytical chemistry in the early 20th century allowed for consistent production and deeper insight, while global industrialization, especially in pharmaceuticals and material science, fueled research into silver lactate’s unique combination of reactivity and solubility.
Silver lactate sits at an interesting intersection between utility and complexity. With relevance in laboratory synthesis, advanced material coatings, antimicrobial treatments, and even niche electronic applications, it's not locked down to a single field. Commercial sources offer silver lactate as a fine white or off-white powder or crystalline solid, selling it both for academic research and for use in product labs working on silver-based innovations. Its availability signals ongoing demand, tied not just to the old guard of chemical synthesis, but to fields like dentistry, antimicrobial polymers, and electrochemistry.
Silver lactate presents itself as a white or faintly yellowish solid, crystallizing well under controlled conditions. It dissolves well in water, thanks to the carboxylate group from lactic acid, making it easier to incorporate into solution-based processes. Molecular formula C3H5AgO3 tells a straightforward story: a single silver ion chelated by a lactic acid-derived anion. The melting point hovers in the moderate range, but more impressive is its chemical responsiveness—silver lactate reacts rapidly with halides and light, forming elemental silver or insoluble silver salts. The compound’s hygroscopic nature calls for moisture-tight storage, and since it breaks down under bright light, containers need to shield it from ambient exposure.
Quality labs define silver lactate by high purity thresholds—often 98% or better—supported by infrared spectroscopy, elemental analysis, and loss-on-drying measurements. Labels on reputable commercial supplies declare percent purity, storage conditions (typically below 25°C, light-protected, and desiccated), recommended shelf life, and hazard pictograms for toxicity and environmental risk. Packaging sizes range from a few grams (for research) to hundreds of grams (for industrial use), each batch accompanied by data sheets spelling out molecular weight (182.95 g/mol), appearance, CAS number (usually 5787-93-9), and supplier-specific batch identification.
Classic synthesis relies on reaction between a soluble silver salt—most often silver nitrate—and a solution of sodium or potassium lactate, with lactic acid neutralized beforehand. Dropwise addition and continuous stirring create conditions for silver lactate to precipitate cleanly. One must filter and wash the precipitate thoroughly to remove nitrate and alkali residues, then dry it under vacuum over desiccant. In modern preparations, ethanol or cold water serves to minimize product loss and increase the crystal yield. Every step prioritizes light avoidance and minimal air exposure to stop decomposition or colloidal silver formation. Some research operations substitute lactic acid and silver oxide, but this route produces less consistent results if the lactic acid's optical purity wavers.
Silver lactate steps up as a lively player in several chemical processes. Light or heat can cause it to decompose, depositing elemental silver. Mixing it with halide solutions generates silver halides, a foundation for classic photographic processes. In complexation reactions, the lactate’s carboxylate group can coordinate with other metal ions, leading to sizeable organometallic assemblies. Chemists harness this reactivity to design silver-based catalysts or to build antimicrobial agents that loosely release silver ions. There's also interest in modifying the lactate backbone itself; chemically altering the hydroxy or methyl groups opens possible paths to new polyol-silver frameworks or unique silver-based coordination polymers. In reduction settings, ascorbic acid or polyols draw metallic silver out of solution, leaving behind free lactate and rich silver nanoparticles.
In chemistry literature and trade catalogs, silver lactate turns up under names such as silver(1) lactate, lactate silver salt, argentous lactate, and often simply as silver lactic acid salt. Catalogs reference CAS 5787-93-9 and the formula AgC3H5O3 for traceability. In various languages, the key identifiers stay close to these English versions, with only the order or the translation of “lactate” signaling regional differences.
Handling silver lactate demands some respect for its toxicity and environmental hazards. Skin or eye contact can irritate, and dust inhalation should be dodged. The compound’s reactivity means spills need prompt cleaning, preferably with gloves and through waste disposal by licensed handlers. Lab protocols require airtight and opaque containers, fume hoods for large-scale operations, and personal protective equipment—lab coats, gloves, and goggles. Waste streams containing silver lactate get managed according to national and local environmental regulations, aiming to keep silver out of waterways, as silver ions threaten aquatic organisms even at low concentrations. Safety data sheets from top manufacturers provide risk codes and first aid advice for accidental exposure.
Silver lactate rides on the back of silver’s unique chemistry, stepping up wherever controlled silver ion release, water solubility, or organic compatibility matters. In healthcare, it’s part of antimicrobial coatings on wound dressings, staving off infection without feeding resistance. Food packaging researchers see promise in incorporating silver lactate into biodegradable films. Dentists and oral care developers tap its potential for cavity prevention and root canal sterilization, leveraging silver’s time-tested antibacterial bite. In material science, it finds use in synthesizing conductive inks for printed electronics, providing a water-soluble silver source that prints, dries, and converts into a conducting trace. Beyond the lab, folks working in environmental detection harness its ability to pull out or sense halides in water samples.
Silver lactate research pulls in several directions. Biomedical engineers dissect its interaction with cell membranes, comparing effectiveness with other silver compounds in killing bacteria but sparing human tissue. Materials scientists push forward on nanoparticle synthesis routes that maximize yield, reduce cost, and control particle size. Analytical chemists work on better detection and recovery of silver ions, vital for environmental safety and precious metal recycling. Each area benefits from collaboration—shared data on toxicity, reactivity, and breakdown products leads to smarter design for next-gen antiseptics or sensors. Fundamental chemistry groups flirt with new derivatives, aiming to tailor degradation rates or optical properties by tweaking the lactic acid skeleton or pairing silver with other functionalized organic acids.
Data on silver lactate toxicity suggest prudence, especially where chronic or widespread use enters the picture. Short-term studies in laboratory animals flag acute irritation and possible respiratory effects at high inhalation levels; longer-term or oral dosing can affect organ systems as silver accumulates. Researchers track argyria—skin turning blue-gray—as the classic marker of silver poisoning from overexposure. Water systems must stay monitored, since silver harms algae and invertebrates at low concentrations. Human cell line studies focus on cytotoxicity and DNA effects, measuring doses that kill bacteria but leave mammalian cells functional. This vigilance keeps silver lactate as an asset rather than a liability, supporting responsible deployment in environments where benefits clearly outweigh the risks.
Silver lactate walks a line between established practice and emerging potential. While traditional uses in antimicrobials and analytical labs continue, new research points toward medical device coatings, advanced diagnostic platforms, and next-generation conductive materials. Environmental sustainability looms larger—recovering silver from used materials fits well with life-cycle management trends, especially if researchers further refine safe breakdown and recycling routes for lactate-based compounds. Synthetic biology groups even explore biosourced lactic acid analogues for greener silver salt production. The evolution of regulatory frameworks and increased scrutiny of silver’s environmental footprint will shape how widely silver lactate appears in future products. The chemistry community’s ongoing investment in precision, safety, and innovation means silver lactate won’t be fading into the background soon.
Silver lactate comes up in some surprising places, especially for those who spend little time poking around in labs or materials science. With its roots planted in both chemistry and medicine, this silver-based compound pulls its weight well beyond a niche role.
Ask anyone who’s worked with bacteria: keeping things clean takes science, not luck. Silver lactate steps in as a bacterial combatant. Researchers add it to petri dishes and agar plates because silver ions break down microbial cells with impressive consistency. In microbiology labs, this cuts contamination, saving both time and effort. Hospitals and research centers pick up on the benefit, especially where resistance to traditional antibiotics grows.
Materials scientists have long chased metals with unique blendable properties. Silver lactate dissolves in water and offers a controlled way to get silver into different compounds and surfaces. This brings options that elemental silver just can't touch. Printing highly conductive inks and pastes stands as one of the most practical examples, especially in electronics. Flexible circuit boards for wearables or smart tags draw on the conductive properties of silver supplied in a manageable form.
In my early research days, silver nitrate ruled the roost as a silver supply, but the byproducts and side reactions got messy fast. Shifting to silver lactate kept equipment cleaner and brought better consistency to experimental outcomes. This isn’t just a matter of convenience; it saves cost and cuts down on hazardous leftovers. These details matter when every line item and result undergoes scrutiny.
Silver's medical use stretches back centuries. Today, silver lactate crops up in wound care materials, coating medical devices to slow or stop infection. With antibiotic resistance rising, this kind of technology draws growing interest. Early studies suggest silver lactate's antimicrobial effects outlast simple silver salts, opening new chances for safer hospital stays and fewer repeat visits. Evidence continues to build, with peer-reviewed research from journals like Applied Microbiology giving the idea weight.
No compound escapes scrutiny, especially those destined for medical or consumer products. Having worked in both academic and industry settings, I’ve seen how regulatory bodies like the FDA and EPA watch silver compounds. Silver lactate meets stricter limits for toxicity and environmental impact than many older silver chemicals. This points to a future where function doesn’t always have to come at the cost of safety.
Cost still plays a role. Silver compounds aren’t cheap, so industrial-scale uses like water purification and textiles move slowly compared to lab work. Ways to recycle and recover silver from waste streams will mark the next big leap—letting companies cut expenses while limiting environmental impact. Smart design, robust research, and responsible handling make all the difference.
Silver lactate’s value stretches past clean test tubes or gleaming surgical tools. Its blend of antimicrobial power, process versatility, and relatively low risk brings hope for real improvements. Choosing solutions supported by both science and personal experience pays off in quality and peace of mind.
Silver always drew attention for its antibacterial punch. Hospitals have used silver dressings for burns and wounds. Over-the-counter gels with silver act as infection fighters. Not every silver compound plays nice with the body, though. Silver nitrate, for example, can damage tissue if not handled carefully. Silver lactate doesn’t get the same spotlight as colloidal silver or silver nitrate, which means research on it remains scarce.
Mixing silver with lactic acid creates silver lactate. Chemists use it in labs, and a few patents mention it as a possible ingredient for antimicrobial creams. There’s nothing mainstream—no FDA-approved silver lactate creams fill local pharmacies. No big-name skin product lists it on the label either. Most references speak to test tube results, not widespread human use.
No one can point to a headline that reads, “Silver Lactate Found Safe for Skincare” or “Study Approves Silver Lactate For Oral Use.” The real trouble starts with how the body reacts to silver overall. Small, short-term exposure—think wound dressings or topical creams—generally stays safe for healthy skin. Eating or inhaling silver is a different story. Too much silver in the body turns skin a bluish-gray, a condition called argyria. The World Health Organization notes that silver on wounds causes few problems, but long-term use or ingestion leads to risk.
Digging through scientific journals, very little shows up about silver lactate and people. Animal studies exist, but those don’t always predict what happens in humans. In a handful of cell culture studies, silver lactate stopped some bacteria from growing. The silver ion part does most of the work there, and any silver compound must be studied for how it breaks down and what parts get absorbed. No one stands behind silver lactate in the same way that scientists or regulators approve silver sulfadiazine for burns.
Doctors and pharmacists stick with what’s proven. Silver sulfadiazine, thanks to rigorous testing, shows both safety and benefit for burns. Silver lactate’s research track record falls short. Consumers—often looking for powerful, natural-sounding germ fighters—deserve real data. People can’t tell if silver lactate causes skin irritation, allergic reactions, or worse, without detailed studies. Food and Drug Administration (FDA) and European Food Safety Authority (EFSA) databases offer no green light for silver lactate in medicine, supplements, or food.
Companies that want to use silver lactate need to support research, sharing both risks and benefits. Transparent studies, published in peer-reviewed journals, matter more than slick advertising. If a researcher aims for safe use, every batch used for people has to meet purity standards, go through animal tests, and receive ethical clearance for clinical trials.
Health agencies track safety with careful review and ongoing monitoring. If silver lactate ever makes it to commercial health products, consumers should have full access to safety data and public research. Companies, regulators, and scientists have to communicate with honesty—using real numbers, not unfounded claims about microbes or “next-generation” cures.
With silver lactate, caution stays on the menu. Nothing replaces patient safety or honest science. Any new ingredient deserves evidence that meets real-world standards—because people want cures, not complications.
Many people don’t realize that careless storage of chemicals can create dangers both for personal health and the stability of the substance itself. Silver lactate stands as a good example. Used in research, engineering, and certain medical applications, this compound doesn’t take well to a sloppy environment. Based on years handling similar chemicals, it’s clear that proper storage is more than a box-checking exercise. It's an important measure for safety and efficiency.
This chemical isn’t just another bottle on the shelf. Silver tends to react, and lactates have tricks of their own. Water vapor from the air, bright light, and traces of ordinary table salt can speed up its breakdown. Once degraded, it not only loses its original purpose, but small amounts of gas and unusual residues can form, which can hurt experiments and pose new dangers. A lapse in the right storage method, even for a day, might damage a whole batch.
Getting lazy about storage could leave someone with a mess—brownish compounds, odd smells, unexpected reactions. Researchers who have seen this firsthand talk about ruined sample runs and extra cleanup time, both of which waste effort and funds.
People sometimes look for complicated tricks, but in the lab, the best answer tends to be the simple one. Silver lactate stores best in airtight glass containers. Plastic tends to let water vapor sneak in and isn’t always fully airtight—something anyone who's ever found a crystallized powder at the bottom of a plastic tube will have seen. Glass jars keep the moisture out, limiting unwanted reactions.
Keep these containers in a location far away from direct sunlight. Sunbeams can change silver at the molecular level, making it clump or stain the container walls. Even a brief exposure to strong light can spell trouble, so a dark cupboard or drawer, well away from windows, helps a lot.
Heat can spell disaster. A shelf near a radiator or in a hot warehouse corner could turn pure product sluggish and sticky. Store it at room temperature, but no more than 25°C (77°F). Most people in lab coats don’t carry around a thermometer, but keeping silver lactate in a room where people feel comfortable proves good for the compound, too.
Silver lactate draws unwanted attention from water and acids. Avoid placing it near sinks, cleaning products, or anything that might emit fumes. Using a sealed secondary container, maybe a sturdy plastic box with a closed lid, reduces the risk from accidental spills or chemical leaks in storage spaces shared with acids or bases.
Relying on memory rarely works out in a busy workplace. Every bottle needs a clear, durable label showing the date received and the date it should be checked. It never hurts to set a reminder—once every three months works well in my experience. If you see signs of clouding, clumping, or any odd color change, play it safe and dispose of the batch following local hazardous waste guidelines.
Good storage habits protect both people and products. Simple, clear rules matter more than fancy tech or overly complicated systems. Those who treat chemical storage as a routine part of their work routine find fewer surprises, save resources, and stay safer.
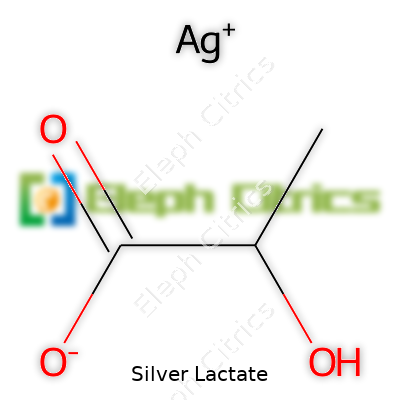
Silver lactate brings together two words that spark curiosity—silver, the well-known metal with a rich history in medicine, and lactate, a compound often associated with the human body or pickled foods. In chemistry circles, people often ask about the formula, but knowing the makeup gives more than a simple set of characters. Silver lactate carries the formula AgC3H5O3. The 'Ag' stands for silver, shining through as the only metallic element in the mix. The lactate part, C3H5O3, comes from lactic acid, the same stuff that builds up in your muscles during a sprint.
Sometimes formulas like these seem locked away in textbooks, isolated from daily life. My background in science taught me the real world shows up in unexpected places—medicine, water disinfection, and even photography. Silver compounds don’t just sit on shelves. AgC3H5O3 plays a role in wound care thanks to the antimicrobial properties of silver. Back in my college lab days, we worked with silver salts, and their germ-killing abilities weren’t some chemistry trivia—they made a difference in patient care. Silver lactate takes part in laboratory tests, helps form thin films in electronics, and pops up in research seeking alternatives to standard antibiotics.
Silver lactate’s formula speaks to the combination of one silver atom for each lactate ion. This balance—one to one—makes it possible to dissolve in water more easily than metallic silver itself. Sources like the PubChem database and the Merck Index demonstrate that the formula stands as AgC3H5O3, confirming its molecular structure across peer-reviewed studies. Sifting through these details stops misinformation in its tracks, which builds trust in science education and makes a difference in laboratory safety.
Despite its positives, silver lactate can cause headaches in handling or disposal. Silver ions react sharply with light and can leave behind stains or even environmental harm if the waste enters water systems. Stronger regulation around disposal, clear labeling, and training can lower these risks. Regulatory agencies like the Environmental Protection Agency (EPA) offer guidelines to keep silver out of drinking water, so following their standards isn’t just red tape—it keeps people and wildlife safe.
Pricing is another issue. Silver compounds aren’t cheap. For smaller labs or developing countries, affording these materials takes careful budgeting. There’s room for innovation—finding recycling methods or more efficient synthesis routes can save costs and support sustainability goals. Research groups who share best practices and open-access methods globally can lift up those with fewer resources.
Chemistry doesn’t live alone in the pages of a manual. The formula AgC3H5O3 isn’t just trivia for students or professionals. It’s a gateway to safer hospitals, cleaner products, and smarter technology. With better training, responsible disposal, and a willingness to share knowledge, the story of silver lactate grows from a formula into a tool for everyday progress.
Anyone buying silver lactate knows there’s more to it than simply picking a bottle off the shelf. Every chemist, researcher, and manufacturing technician keeps a close eye on one thing above all: purity. In labs and industrial set-ups, the purity of a chemical can make all the difference between a successful experiment and a waste of time and money. Silver lactate draws its value from silver content and how free it is from contaminants like lead, mercury, or other ions that turn a trusted compound into a potential hazard.
Purity grades can run from technical grade, often around 95% or so, up through ACS (American Chemical Society) or reagent-grade, which means at least 99% or higher. In my own hands-on work, nothing throws off results quite like traces of sodium or iron sneaking into what’s supposed to be pure silver lactate. Researchers using it in electronic applications or sensitive pharmaceutical experiments can’t risk these extras showing up.
Here’s something often overlooked: regulations don’t just recommend top purity—they demand it. Regulatory agencies in the US, Europe, and Japan have clear standards, especially if the compound enters medical or food-grade territory. Meeting these rules takes rigorous testing. I remember handling a batch marked as 98.5% pure, only for a project partner’s test to spot a spike in calcium. That small difference could have ruined a whole week’s work if we hadn’t double-checked with our own analysis.
Poorly labeled silver lactate causes headaches for vendors and buyers. About a decade ago, there was an incident in a manufacturing plant where the label claimed “over 99%” but failed to note the presence of nickel. The result? Several thousand dollars lost in faulty product runs and some heavy fines after a safety audit. Since then, transparency in labeling and traceability became absolute priorities in our sourcing. Everyone in the chain deserves real numbers with their chemicals—not vague guesses.
Most reputable suppliers rely on high-performance liquid chromatography and atomic absorption spectroscopy. These aren’t just fancy terms—they are bread-and-butter tools for any testing lab. They catch even tiny variations in silver content or foreign ions, acting as the backbone for any claim about grade. Many catalog entries now come with downloadable Certificates of Analysis. In daily practice, I won’t accept a sample unless I see that certificate, preferably with batch-specific data instead of broad averages.
Bigger buyers tend to work out supply contracts that spell out batch-by-batch testing, spot audits, and routine third-party checks. It comes down to trust earned by real documentation, not empty promises. More labs are starting to demand this extra layer—especially anyone working near regulated markets or where performance matters as much as price.
If suppliers want to stay relevant, they must stay open and fastidious about their grades. Silver lactate with a strong analysis backing it up doesn’t just fill a bottle—it builds confidence, protects results, and makes sure every dollar spent gives back the reliability real work demands.
| Names | |
| Preferred IUPAC name | silver;2-hydroxypropanoate |
| Other names |
Silver(I) lactate Lactic acid silver salt Silver lactic acid salt |
| Pronunciation | /ˈsɪlvər ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 12125-21-2 |
| Beilstein Reference | Beilstein 3749221 |
| ChEBI | CHEBI:9186 |
| ChEMBL | CHEMBL504539 |
| ChemSpider | 20568871 |
| DrugBank | DB11061 |
| ECHA InfoCard | 100.040.327 |
| EC Number | 208-238-1 |
| Gmelin Reference | 1143 |
| KEGG | C18612 |
| MeSH | D015245 |
| PubChem CID | 157308 |
| RTECS number | OW2650000 |
| UNII | 099G6S469G |
| UN number | UN1325 |
| CompTox Dashboard (EPA) | DTXSID60873242 |
| Properties | |
| Chemical formula | AgC3H5O3 |
| Molar mass | 230.94 g/mol |
| Appearance | White powder |
| Odor | Odorless |
| Density | 2.50 g/cm3 |
| Solubility in water | Soluble |
| log P | -2.32 |
| Acidity (pKa) | 7.8 |
| Basicity (pKb) | 12.6 |
| Magnetic susceptibility (χ) | -22.0e-6 cm³/mol |
| Refractive index (nD) | 1.57 |
| Dipole moment | 0 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 155.8 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -789.65 kJ/mol |
| Pharmacology | |
| ATC code | D08AL01 |
| Hazards | |
| Main hazards | Harmful if swallowed. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Precautionary statements: "P261, P264, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362+P364, P501 |
| NFPA 704 (fire diamond) | 1-2-0 |
| Lethal dose or concentration | LD50 (oral, rat): 1830 mg/kg |
| LD50 (median dose) | LD50 (median dose) for Silver Lactate: 259 mg/kg (rat, oral) |
| NIOSH | GRY |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Silver Lactate: "0.01 mg/m3 (as Ag) |
| REL (Recommended) | 120 mg/L |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Lactic acid Sodium lactate Potassium lactate Calcium lactate Silver acetate |