Sodium citrate’s story stretches back to the days of early industrial chemistry. Citric acid itself, first isolated from lemon juice in the late 1700s, set the stage for its salt derivatives. Technology caught up in the 19th century, making it possible to extract not just the acid but create sodium citrate through straightforward neutralization. Food preservation pushed a lot of these advances—people wanted fruit flavors in winter, milk that stayed fresh a little longer, and canned goods that stayed palatable. During World War I, sodium citrate helped propel blood transfusion science. Doctors learned it could prevent blood from clotting outside the body, making stored blood viable for longer and directly saving lives.
Sodium citrate works as a buffering agent, preservative, and sequestrant, shifting the balance in products from cheese to pharmaceuticals. In food processing, it keeps flavors balanced and blocks nasty aftertastes from metal ions. In the lab and in hospitals, it stands out as an anticoagulant, vital for stored plasma and routine blood analysis. The three-sodium version—trisodium citrate—delivers the most power per gram, but mono- and di-sodium citrate make appearances depending on acidity needs. This flexibility keeps sodium citrate useful across industries, from fizzy drinks to dialysis machines.
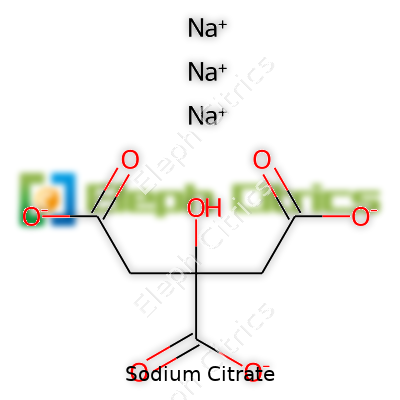
Sodium citrate stands out for its clear, crystalline appearance, dissolving readily in water to create a slightly basic solution. It doesn’t carry pungent smells or leave residues, which helps with food and drink use. The pH-raising effect of a sodium citrate solution becomes crucial in dairy—and this is why it prevents curdling in cheese sauces. Its melting point sits around 300°C, so it endures high-heat food processes without breaking down or losing functionality. Its molecular formula—C6H5Na3O7—lays out a six-carbon backbone with three sodium atoms replacing the original hydrogen groups from citric acid.
In food and pharma, sodium citrate specifications matter for purity, moisture, and trace elements like heavy metals. The United States Pharmacopeia and European Pharmacopeia provide clear standards—most products test above 99% purity. Labels must list sodium citrate components, including amount and grade (e.g., 'food grade,' 'pharmaceutical grade'). Food safety regulations in the U.S. (FDA) and Europe (EFSA) enforce maximum usage levels and labeling language. These standards keep allergic reactions rare and mislabeling risks low.
Production leverages simple chemistry. Citric acid, often derived from fermented sugars using mold cultures, reacts directly with sodium carbonate or sodium bicarbonate. The byproducts—often water and, depending on the base, carbon dioxide—drive the reaction to completion. Processors then evaporate the water to leave fine, white crystals. These crystals can be milled into different sizes or spray-dried for applications that need easier mixing. Controls at each stage cut down on contamination and residual solvents, fetching higher quality for medical or high-end culinary use.
Sodium citrate doesn’t just stay put, chemically speaking. In water, it can lose sodium ions and shift local pH. That property blocks calcium and magnesium from driving unwanted deposits in food and industrial processes. Researchers tailor its sodium-to-citrate ratio to tweak buffering capacity. Some labs even explore loading the molecule with other metal ions for specialized chelation tasks, such as pulling toxic metals from bloodstreams or cleaning up industrial wastewater. These new complexes could serve as anti-coagulant substitutes or work in environmental cleanup.
The chemical literature—and supermarket labels—reference sodium citrate by a tangle of names. Look for 'trisodium citrate,' its E-number E331, or sometimes just 'citric acid sodium salt.' Pharmaceutical cartons may say 'blood anticoagulant solution.' In cleaning products, it might appear as a 'water softener.' All these point back to sodium citrate’s same core structure, but ingredient lists respond to regulatory requirements and consumer familiarity. Chemists and engineers scan for CAS numbers (68-04-2 for trisodium citrate) to avoid mix-ups, since a change in sodium content can mean a big shift in function.
Routine handling of sodium citrate falls under basic food- and pharma-grade protocols. Dust control, glove use, and closed-system processing limit inhalation and skin contact. It doesn’t pose high risks in standard conditions and shows up on food safety regulatory lists as 'generally recognized as safe' (GRAS) in the U.S. Employees learn spill cleanup and use eye protection, though the dust causes little more than mild irritation. Chronic overuse in foods could edge sodium intake higher—fueling heart and blood pressure concerns—but the compound doesn’t present hazard risks like many synthetic additives. Warehouses stick to dry, tightly sealed packaging to stop clumping and preserve product stability.
Sodium citrate shapes an impressive range of fields. Food technologists rely on it for flavor stability and creamy foundations in cheeses and desserts. Beverage makers add it for tartness and fizz control. Hospitals couldn’t function without sodium citrate in their blood bags—this single compound allows blood banks to function smoothly. Home cooks reach for it to nail the texture of melting cheeses for mac-and-cheese. Water treatment specialists dose it to curb scale buildup, cutting costly equipment repairs and extending lifespans of industrial boilers. Pharmaceutical researchers develop syrup bases with sodium citrate for easier swallowing and better shelf life. It also plays roles in dental products to buffer acidity and protect enamel.
Labs worldwide keep pushing sodium citrate’s ceiling. Scientists test new delivery forms for kidney stone prevention and optimize its use in oral rehydration salts for children with diarrhea. Food scientists develop dairy-free cheese textures that rival the real thing. Engineers ask how it might help new processes in battery manufacturing, since its chelation properties can stabilize heavy-metal ions during recycling. Ongoing studies probe combinations with other bioactive salts and look for ways to improve environmental outcomes, such as biodegradation post-use and more sustainable feedstocks for citric acid fermentation. Some are studying its effects in metabolic disease panels, aiming for better treatments with fewer side effects than conventional sodium therapies.
Regulators and researchers agree on sodium citrate’s low acute toxicity. Rat studies show survival even at high doses, and decades of clinical use in blood storage and medicine confirm this record. That being said, high intake over time—through processed foods—can escalate sodium levels, raising risks for hypertension and heart disease. Citrate’s natural role in the body’s metabolism makes most people tolerant of it, but rare metabolic disorders (such as citrate metabolism problems) call for caution. Safety rules focus more on managing cross-contamination from allergens, keeping non-food-grade batches away from edible applications, and tracking batch purity. Researchers keep an eye on long-term effects, but so far, the real health threats tend to come from excess sodium as a broad public health concern, not from citrate itself.
The future for sodium citrate looks bright, with scientists targeting smarter uses in targeted medicine, waste cleanup, and plant-based food revolution. Trends in ultra-processed food draw scrutiny, but sodium citrate’s role as a relatively mild, well-studied ingredient places it ahead of riskier and less-researched additives. Novel production methods using genetically tuned mold or yeast could lower costs and environmental burdens, while tweaks in product purity will open new doors for medical and electronic applications. Efforts to cut global sodium intake will likely push formulators to use lower doses or pair sodium citrate with potassium citrate, keeping flavor and function intact while helping public health. Players across food, medicine, and environmental sectors keep finding new ways to put this versatile old-timer to use—proof positive that humble molecules can still make a world of difference.
Many people run into sodium citrate in food ingredient lists before ever realizing its reach. Its name pops up most in processed cheese, powdered drinks, and club soda. Take processed cheese, for example. Sodium citrate lets cheddar or Swiss turn silky smooth when melted, instead of breaking into oil and clumps. It’s what keeps macaroni and cheese sauce glossy instead of greasy.
In my own kitchen, I’ve used sodium citrate to rescue homemade cheese sauce more than once. Toss a bit into the milk and cheese, and suddenly the mixture goes creamy like a pro chef finished it. Some home cooks swear by it for smoothing out homemade “velveeta” and even ice cream mixes. That consistency comes with real science: sodium citrate controls pH, stopping proteins from tangling up and separating.
Hospitals and clinics keep sodium citrate stocked for reasons far beyond flavor. Doctors use it in blood transfusions because it binds to calcium and slows clotting, which means blood can sit in bags or tubes a bit longer before use. It also comes into play for people at risk of kidney stones. Nearly half a million Americans land in emergency rooms from kidney stones yearly, and citrate salts help by curbing crystal formation in urine.
I’ve met folks dealing with chronic kidney stones, and recurring pain pushes them to search for any relief. It’s easy to overlook humble sodium citrate on a prescription bottle, but it can genuinely improve daily quality of life for these patients. On a related note, some antacids combine sodium citrate with other ingredients to tame heartburn, working on that same principle of pH balance.
People worry about food additives, often with good reason. Research shows sodium citrate breaks down into sodium and citrate naturally present in food and the body. Still, sodium gets tricky in a diet. Packaged foods already push salt levels into ranges that health experts tie to high blood pressure and heart disease. The FDA considers sodium citrate “generally recognized as safe,” but that doesn’t mean every individual needs more sodium in their diet. For those watching salt, processed food with sodium citrate can tip the daily total over the recommended limit fast.
Medical uses come with safeguards. Hospitals monitor patients carefully, so overdose or imbalance rarely happens in these environments. Out in the world, self-medicating for things like kidney stones or heartburn needs caution. Too much can upset the body’s natural mineral balance and cause side effects like nausea or twitching muscles. Bringing a doctor into the conversation before starting new supplements makes a smart move, especially for people with a history of heart or kidney problems.
There’s nothing wrong with sodium citrate as a tool. Its strengths show up most when used deliberately, not automatically. Food companies could focus more on taste and stability from real ingredients, and cut down on how much sodium lands in a single meal. Personal health tracking can help people make better choices about the food and supplements they use. Awareness and balance matter more today than ever, as just about everything in the grocery store has something added to it.
Sodium citrate carves out a helpful spot in both food and medicine. What matters is recognizing its effect, using it wisely, and thinking twice about whether more “smooth and creamy” is worth the salt that comes with it.
Sodium citrate pops up a lot on ingredient lists, especially in processed cheese, sodas, and powdered drink mixes. Think of it as the thing cheesemakers trust to keep cheese gooey in nachos and macaroni. Companies also use it to control acidity in drinks and to help preserve shelf life. The chemical comes from citric acid, found naturally in citrus fruits, but most sodium citrate comes from fermenting starches with certain bacteria and then combining the result with a little sodium.
Food safety means a lot to families, chefs, and folks like me who want to know what’s going into our kids’ lunches. Sodium citrate gets stamped as “Generally Recognized As Safe” (GRAS) by the U.S. Food and Drug Administration. In real terms, that’s not just a rubber stamp handed out for anything. Regulators actually review research and experience from food scientists before handing out that label.
Studies covering both animals and people show that sodium citrate doesn’t pile up in the body. Our systems break it down, just like natural citrate from oranges or lemons, and flush out what isn’t used. Some research does look at its use in medicine too, where it helps treat conditions like kidney stones or as an anticoagulant. Even at clinical doses, which run higher than what’s in food, side effects usually involve mild stomach upset or diarrhea.
While most nutrition experts don’t single out sodium citrate as a health villain, the bigger story involves how often we eat ultra-processed foods. Many people eat more processed cheese, instant puddings, or fizzy drinks than they realize. Even if sodium citrate on its own isn’t setting off alarm bells, eating heavily processed foods often means taking in too much sodium in general. Extra sodium can raise blood pressure over time. The CDC points to high blood pressure as a leading risk for heart disease in the United States—a problem that affects millions.
I’ve seen families get caught off guard. They think skipping table salt and switching to packaged “low-sodium” options keeps everything in check. Turns out, the sodium in stabilizers or preservatives like sodium citrate quietly pushes the total higher, even if single servings look harmless on paper.
Checking ingredient labels makes a difference for anyone watching sodium levels, whether because of doctor’s advice or just wanting to eat better. I like using the “less-processed plate” trick: fill most meals with fresh veggies, whole grains, and proteins that don’t come wrapped in plastic. That means sodium citrate, if it shows up at all, ends up as a small part of your diet.
Ultimately, most healthy adults can eat foods with sodium citrate without worrying. Parents sometimes worry about all the strange ingredient names. In my experience as someone who loves a gooey cheese dip, moderation works—just don’t let convenience foods push out too many home-cooked basics. If you have high blood pressure, kidney problems, or need a low-sodium diet, work with your doctor or a registered dietitian: they’ll help spot hidden sources and give you practical tips for meal planning.
Food chemistry keeps evolving, but common sense still goes a long way. Sodium citrate gives us melty cheese sauce for a weekend treat or a tartness boost in a sports drink. Eating it doesn’t cause problems for most people. The bigger issue still comes down to how we build our meals, not just what ingredient sits at the bottom of the label.
People use sodium citrate for a bunch of different reasons. Hospitals keep it around to stop blood from clotting during transfusions. Cooks rely on it to help cheese melt smoothly, so nacho night doesn’t turn into a disaster. Some athletes even take it to deal with acid build-up during intense workouts. With so many uses, questions about its safety keep coming up. I’ve seen both the benefits and concerns play out in real-life situations.
Doctors and pharmacists know that sodium citrate can mess with the body a bit. Folks sometimes notice stomach cramps, gas, or even diarrhea. It doesn’t feel good to have to run to the bathroom after every meal or snack that included processed foods. Heartburn can sneak up, too. For most people, these stomach troubles go away after the body adjusts or when they stop using products with sodium citrate.
Some people deal with more serious issues. Those with heart or kidney problems should pay close attention. Sodium citrate can raise sodium levels, making it tough for the body to keep up with all the extra salt. Too much sodium spells trouble for blood pressure and can burden the heart. People who already face congestive heart failure or chronic kidney disease end up feeling a lot worse if sodium isn’t watched closely.
Older adults and anyone taking medications that affect electrolyte balance face even more complications. Potassium and calcium levels might get out of whack, and muscle cramps or irregular heartbeats can show up. Emergency rooms sometimes see patients who were just trying to relieve acid reflux and ended up with muscle weakness or confusion.
People forget how many processed foods list sodium citrate on the label. From canned soups to energy drinks, it sneaks into foods most folks wouldn’t expect. Those with high blood pressure, on restricted sodium diets, or living with kidney disease need to check labels more often, even on snacks that seem innocent. Eating out complicates things, since restaurants don’t usually tell you what’s hidden in the sauce or cheese.
Medical journals and the U.S. National Library of Medicine spell things out. Mild stomach issues top the list, but serious reactions remain rare for healthy people. For patients who already retain too much sodium or those on certain heart medications, even a modest increase can tip the scales. Take a case where a patient treated for kidney problems developed edema—swelling in the legs—after consistently consuming foods packed with sodium-based additives. Monitoring blood sodium told the real story.
Anyone uncertain about sodium citrate’s impact on their body should talk with a healthcare professional, not just search online or guess. Regular blood work offers real clues about how your body responds. Doctors can spot red flags before things get serious.
Cooking at home with whole ingredients puts control back in your hands. Making cheese sauce from scratch, skipping heavily processed snacks, and reading the back of the box all help. Healthcare teams often remind patients with chronic illnesses to check every label and talk over new symptoms early.
Spending years working with patients and reading ingredient lists myself, it’s clear that sodium citrate deserves respect—useful when handled wisely, risky when ignored. With the right questions and a little caution, most people steer clear of serious trouble.
Sodium citrate sits on the shelf in food labs, hospital pharmacies, and home kitchens. The bag or jar looks harmless—a regular white powder, something between salt and baking soda. Still, proper storage makes a difference, not only for safety but also for keeping the stuff from clumping up or losing its usefulness.
Sodium citrate absorbs moisture from the air. Think of a humid summer day when the sugar in your bowl suddenly turns lumpy. Leaving this chemical open or in a thinly closed bag, it draws in water, setting off little clumps. Clumped powder creates headaches during weighing and mixing, which can throw off recipes and experiments. Beyond the kitchen, any inaccuracies become even more serious in a hospital or research setting.
Shelf life also comes into play. Moisture is the enemy of powders. Once water gets into a container, bacteria and mold see opportunity. This might not cause alarms right away with sodium citrate, but over months, it leads to changes in odor, texture, and safety.
In real life, small things go a long way. Always keep sodium citrate tightly sealed. Move the powder to an airtight jar with a screw-on lid or a thick, heavy-duty zipper bag. Clear, hard-sided containers are best—cheap thin plastic cracks, letting in air and bugs. The less you expose the powder to open air, the longer it keeps its original quality.
Heat turns storage into a big problem. Sticking sodium citrate near a stove, boiler, or sunny window shortens its lifespan. High temperatures set off slow chemical changes and may let condensation form inside the package. Keep the jar in a cool, dry cupboard away from steam, sunlight, or any source of heat. If the room often gets hot, find a spot in a pantry or closet on a bottom shelf instead of an upper one, where warm air gathers.
I once faced a cupboard where every white powder looked the same. To sidestep confusion, label every jar. Write the name and the date you bought or opened it. In professional settings, that label can dodge countless mistakes and protect anyone new to the workspace from grabbing the wrong powder.
In busy kitchens or labs, someone’s always rushing. Scooping different powders with the same spoon can transfer small bits, spoiling results. Reserve a scoop only for sodium citrate. Give the container a shake now and then to check for signs of clumping or a new smell—these suggest moisture made it in, and the powder might need replacing.
Placing a food-grade desiccant packet in the jar defends against moisture. The little packets soak up stray water, prolonging shelf life. In shared spaces, set aside a central, labeled container, and make everyone responsible for keeping it sealed and dry. At home, it pays to buy smaller amounts more often, cutting back on waste and spoilage.
By paying attention to where this ingredient lives and how it gets handled, you help avoid spoilage, mistakes, and cost overruns. It’s routine, but the daily habit of careful storage gives peace of mind and keeps projects moving on schedule.
Sodium citrate pops up often in everyday medicines and processed foods. Doctors use it to help with urinary tract problems and to balance the body’s acids. Grocery shelves stock it as a food additive. Its reputation for safety makes it easy to overlook, but mixing it with other medications sometimes creates unexpected results.
Sodium citrate shifts the body’s acid-base balance by raising pH in the blood and urine. Most people don’t realize this shift can affect how other drugs work. Take pain medicine like aspirin or antibiotics such as tetracycline; they both depend on the acidity of urine for either absorption or removal from the body. Changing the urine’s pH—making it more alkaline—can slow down the exit of some drugs or make them less effective.
Blood thinners such as warfarin and antibiotics like methenamine work best at certain urine pH levels. Mixing these drugs with sodium citrate may lower the drug’s impact or raise the risk of side effects. For example, methenamine needs acidic urine to kill bacteria in urinary tract infections. Make the urine too alkaline, and this medicine loses its punch.
Antacids and diuretics tell a similar story. Combining citrates with water pills might boost sodium levels too high. I’ve seen patients on both medicines come back with swelling and headaches. Safe on their own, these drugs together create a risk that’s easy to overlook in a busy doctor’s office.
Doctors, pharmacists, and patients sometimes miss potential drug interactions because over-the-counter (OTC) products seem harmless. Pharmacies rarely warn about the combinations, and labels stay vague. It took me learning the hard way after a friend ended up with a racing heart and stomach pain from mixing seemingly safe antacids with her daily meds. Only after a review with her pharmacist did the picture become clear.
For people with heart or kidney issues, sodium citrate’s sodium content also deserves attention. Adding more sodium through medicine bumps up blood pressure risk. Folks living with chronic illness or those juggling more than three prescriptions need to double-check all ingredients, not just the active medicine.
People find real value by making open conversations with their health care team part of their routine. Every time a new prescription or over-the-counter item lands in the medicine cabinet, check in with the doctor or pharmacist. List all medications—including dietary supplements and foods commonly eaten—when talking to health care professionals.
The safest step remains keeping a list of all products and sharing it often with both doctors and pharmacists. If unsure, look up interactions in reliable sources like Drugs.com or ask straight out. Hospitals sometimes run a medication check-up, looking for possible problems before trouble starts. Anyone dealing with chronic health conditions—especially kidney, liver, or heart issues—should build this habit.
Sodium citrate might appear harmless on its own, but it sometimes throws a wrench into the works with certain medicines. By staying alert, asking questions, and looping in health care professionals, people can protect themselves and avoid unnecessary surprises.
| Names | |
| Preferred IUPAC name | Trisodium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Trisodium citrate Citrosodine Sodium salt of citric acid E331 |
| Pronunciation | /ˈsəʊdiəm ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 68-04-2 |
| Beilstein Reference | 3586435 |
| ChEBI | CHEBI:6309 |
| ChEMBL | CHEMBL1356 |
| ChemSpider | 13751 |
| DrugBank | DB04077 |
| ECHA InfoCard | 100.007.657 |
| EC Number | 200-675-3 |
| Gmelin Reference | 1437 |
| KEGG | C00735 |
| MeSH | D018491 |
| PubChem CID | 6224 |
| RTECS number | WH0000000 |
| UNII | RMF9TN9U7Q |
| UN number | UN3077 |
| Properties | |
| Chemical formula | Na₃C₆H₅O₇ |
| Molar mass | 258.06 g/mol |
| Appearance | White or almost white, crystalline powder or granules |
| Odor | Odorless |
| Density | Density: 1.7 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | pKa1 = 3.13, pKa2 = 4.76, pKa3 = 6.40 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | -53.0e-6 cm³/mol |
| Refractive index (nD) | 1.439 |
| Dipole moment | 2.56 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 374.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1566 kJ/mol |
| Pharmacology | |
| ATC code | B05CX04 |
| Hazards | |
| Main hazards | May cause mild skin and eye irritation. |
| GHS labelling | GHS07 |
| Pictograms | GHS07 |
| Signal word | No signal word |
| Hazard statements | No hazard statements. |
| Precautionary statements | Keep container tightly closed. Store in a dry, cool and well-ventilated place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 210 °C |
| Autoignition temperature | Autoignition temperature: 745°C (1373°F) |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 (oral, rat): 8,400 mg/kg |
| LD50 (median dose) | > 5400 mg/kg (rat, oral) |
| NIOSH | WXK9697XGA |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Sodium Citrate: Not established |
| REL (Recommended) | 290 – 870 mg |
| Related compounds | |
| Related compounds |
Citric acid Monosodium citrate Disodium citrate Trisodium citrate Potassium citrate Calcium citrate Ammonium citrate |