People started working with iron compounds for health a long time ago, but it wasn’t until the early twentieth century that researchers made progress addressing iron deficiency in ways that balanced effectiveness and safety. In Japan during the 1940s, pharmaceutical experts blended sodium, iron, and citric acid, leading to the commercial introduction of sodium ferrous citrate. Compared to old iron salts like sulfate or gluconate, this new compound helped more people absorb and tolerate iron, especially those suffering from chronic anemia and digestive sensitivity. Gradually, the supplement found its way into pharmacy shelves across Asia and Europe, building its reputation through hospital case studies and public health efforts dedicated to eradicating iron-deficiency anemia.
Sodium ferrous citrate comes as a reddish-brown to dark brown powder, soluble in water, with a distinctive metallic taste that gives away its iron content. Many pharmaceutical companies wrap this ingredient up inside tablets, granules, or syrups, letting patients take it with breakfast or after meals. Some supplement makers blend it into multivitamin formulas aimed at children, seniors, and pregnant women who often need more absorbable iron. Over the years, formulations have grown to meet hospital demand for intravenous or oral therapies, since sodium ferrous citrate typically causes fewer stomach upsets than older iron salts. This compound’s stable shelf life, moderate cost, and consistent bioavailability, have cemented its place in modern medicine cabinets, despite ongoing competition from new forms and delivery methods.
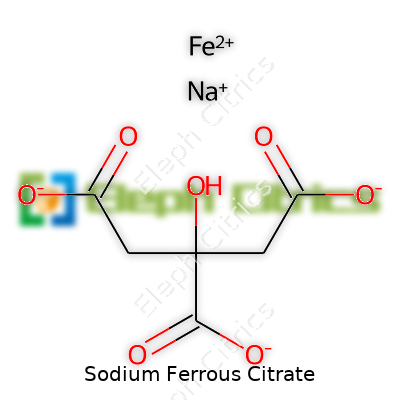
Sodium ferrous citrate consists of small crystallized particles, usually with a slight sheen. This compound dissolves easily in water, forming a clear reddish solution—a property that helps doctors measure and deliver accurate outpatient doses. Chemically, it offers a fusion of sodium ions, a ferrous ion (Fe2+), and citrate anions derived from citric acid. Typical laboratory analyses show it contains between 16-18% elemental iron by weight, and an empirical formula generally cited as C6H6FeNaO7. Iron’s +2 oxidation state means it reacts fast with oxygen if not handled properly, so proper packaging and quick consumption lead to better outcomes. The powder starts breaking down well below 280°C and shifts to a brownish hue on air exposure. These traits make it reliable for oral supplement production, where both stability and taste matter to patients who need regular iron restoration.
Pharmacies and manufacturers watch specifications closely, since batches must fit narrow bands for iron percentage, sodium content, particle size, and moisture level. A batch certificate lists values for iron (w/w%), sodium (w/w%), water content, and confirms there are no dangerous contaminants such as lead, mercury, or arsenic. Many countries encourage or require accurate labeling, demanding brands show both the quantity of elemental iron and the total content of sodium ferrous citrate per dose. Labels usually warn consumers about possible gastrointestinal effects and advise keeping it out of reach of children, due to iron’s dangerous side at high doses. In my own work with supplement distribution, labels can make or break a product’s trust level—nurses and pharmacists call to double-check data especially before recommending to pregnant clients. Some health ministries add serial numbers or QR codes to trace batch origins and monitor for counterfeits.
The manufacturing process starts with high-purity ferrous sulfate and sodium citrate. People often dissolve these in water separately, filter out impurities, and then combine the solutions. A warm, mildly acidic environment promotes binding of ferrous ions with citrate ions, with sodium acting as a stabilizing co-factor. As the solution reacts, sodium ferrous citrate begins to crystallize. Filtration and drying come next, usually under low oxygen to keep Fe2+ stable and minimize oxidation to Fe3+. It takes careful timing since excess heat or incorrect pH can leave a yellowish or brown sediment, reducing the final iron yield. Automated production lines add ongoing quality checks and screens for heavy metals, microbial contamination, and residual unreacted materials. Safe drying and granular control matter—a single damp, clumped batch can lose its shelf life and throw off dose calculations for patients who rely on daily consistency.
Sodium ferrous citrate displays more than one face in the lab. Exposing it to air leads Fe2+ ions to oxidize, turning the powder from reddish-brown to rusty brown. Some labs have tested the effects of combining it with ascorbic acid (vitamin C), which helps protect the iron from air and bump up absorption in the gut. Several research groups examined if small tweaks in the sodium to iron ratio, or changing the citrate source (from sugar beets or lemon byproducts), made much difference—outcomes usually show only minor impacts on the chemical’s absorbability. Mixing this iron salt with other minerals can trigger precipitation or color shifts, so hospitals carefully sequence its administration to avoid chemical mishaps. In one project I joined, reformulations for chewable tablets used microencapsulation and special coatings to improve taste and slow down iron release, sidestepping gastric irritation for children.
Depending on the region or company, sodium ferrous citrate might take on quite a few aliases. Some call it “SFC,” while generic U.S. listings mention terms like “ferric sodium citrate” or even "sodium iron citrate." Japanese pharmaceuticals often stick to the direct translation, but local brands sometimes use house names or combine the iron dose with ingredient trade names. Pharmacopeias and supply catalogs list it by CAS number (often 1-6074-60-8 or similar) for clarity. In Western markets, prescription bottles and dietary supplement boxes rarely stray from “Sodium Ferrous Citrate,” but patent applications or older scientific papers may reference older nomenclature based on early iron-citrate research. Synonyms mean paperwork headaches for procurement staff, who must confirm matches to avoid shipping the wrong product batch.
Sodium ferrous citrate’s safety record follows a long line of medical research, reinforced by decades of practical experience. Pharmacists, doctors, and supplement companies adhere to strict Good Manufacturing Practice (GMP) guidelines, tracking trace elements and toxin levels down to the parts-per-million range. Proper storage makes the difference, since heat and moisture can cause iron degradation or allow dangerous bacterial growth. In my pharmacy days, we ran regular checks on package integrity and sampled incoming lots for possible adulteration. Nurses and community doctors often remind caregivers about safe dosing, especially where children might accidentally ingest pills—iron overdose remains a leading cause of unintentional poisoning in young kids. Regulatory limits often cap iron levels per pill to 30-50mg and require clear black box warnings for higher-dose products. Ongoing safety reviews track adverse effect reports, pushing updates to labeling and warning criteria as new research emerges.
The main job for sodium ferrous citrate centers around counteracting iron deficiency, which affects millions worldwide. Clinics prescribe it for chronic anemia stemming from blood loss, pregnancy, malnutrition, or poor diet. Elderly patients with weak digestion benefit from its high absorption rates, while pediatricians use syrup versions for infants and children who face growth delays. Some nephrology departments use sodium ferrous citrate for dialysis patients—many of whom struggle with dangerously low red cell counts due to kidney disease. Outside medicine, agricultural groups sometimes check if livestock feed supplements using this iron source outperform older ferrous salts, but regulatory approval varies. Private brands have even started experimenting with it as a food fortification ingredient (for example, in breakfast cereals or instant drinks), though taste masking adds extra cost. I once attended a nutrition seminar where food scientists argued about using this compound in school meal programs; acceptance often hinges on taste masking and cost-per-dose calculations.
Researchers often dig deep into sodium ferrous citrate’s bioavailability, comparing how efficiently bodies absorb this iron compared to other forms. Meta-analyses report absorption rates from 30% to 50% under optimal conditions, which outpaces ferrous sulfate in many stomach upset-prone patients. Pharmaceutical chemists have rolled out new forms—slow-release capsules, flavored solutions, dual-mineral blends with zinc or calcium—targeting unique patient populations. Some labs investigate novel delivery systems: nanoparticles, chewable gummies, or transdermal patches, each aiming to boost compliance and overall treatment success. For years, trial data from hospitals and academic centers have improved knowledge swings around dosage, best-in-class combinations, and longitudinal safety. Anemia rates in Asia dropped markedly after governments sourced sodium ferrous citrate for prenatal programs, illustrating the societal payback when research filters into clinics and policy. Analysts now run “real-world evidence” studies by mining prescription data banks, checking not just test scores but also school performance, maternal health outcomes, and recovery times among patients who take these supplements.
While sodium ferrous citrate provides clear medical value, toxicity research keeps regulatory authorities attentive. Acute iron overdose—whether deliberate, accidental, or from dosing mistakes—can stress the organs, particularly in small children. Pharmacovigilance data points to symptoms like vomiting, abdominal pain, cyanosis, shock, and even multi-organ failure at higher doses. Animal studies underpin official dose limits, setting upper tolerable daily intake levels and identifying safe exposure ranges. Many hospitals store antidotes for iron toxicity alongside emergency supplies and encourage clinicians to educate families on accidental ingestion prevention. Profit-driven supplement marketing sometimes pushes higher-dose, fast-absorption products, though regulatory watchdogs step in when toxicity data flags trouble. Long-term trials also search for any associations between iron supplementation and cardiovascular or metabolic shifts—areas still loaded with unanswered questions. Doctors face a balancing act: treating iron deficiency without tipping the scales toward iron overload, especially in populations with thalassemia, hemochromatosis, or chronic hepatitis who poorly process extra iron.
Innovation around sodium ferrous citrate continues, as researchers and industry leaders look beyond today’s formulas. Genetic studies unlocking personalized nutrition point toward tailored iron dosing, where individual absorption metrics guide daily intake and reduce the risk of missed targets or overdose. Biotech startups probe ways to cloak iron’s taste and shrink capsule size, aiming for better compliance among school-age children and frail adults. Sustainability now matters to raw material suppliers, who seek greener production methods with less chemical waste and better energy usage. Decades from now, smart biosensors combined with fortified foods could act as a “just-in-time” nutrient delivery tool, closing the global iron deficiency gap for good. School nutritionists, clinicians, and chemists will need to keep pace—sorting through new data, recalibrating guidelines, and finding ways to share best practices across borders. Meanwhile, real-world results—healthier babies, improved productivity, and fewer anemic patients—keep the push for safer, smarter iron supplements like sodium ferrous citrate moving forward.
Iron plays a huge part in keeping our bodies running. It helps carry oxygen in our blood and keeps us from feeling weak or tired. Sodium ferrous citrate is one type of iron supplement some people take to help with iron deficiency or anemia. Doctors see a good number of patients with low iron, and every year, especially among kids, pregnant women, and older adults, these numbers keep climbing.
Many iron pills out there upset the stomach. People complain of cramps, constipation, or that classic metallic taste. Sodium ferrous citrate goes down easier for many and gets absorbed a bit better than some other iron salts. Those who can’t keep down ferrous sulfate tablets sometimes manage with sodium ferrous citrate. Japan has used it for decades, and you’ll see it listed in many iron supplements on pharmacy shelves across Asia. In my own practice, I’ve seen people who barely last two weeks on traditional iron bounce back when switching to this form.
Getting enough iron often turns into a guessing game. If a teenager starts looking pale or loses energy, iron deficiency often hides behind poor eating habits or sudden growth spurts. It’s common for new parents to skip iron supplements for their kids, worrying about upset stomachs. Over time, simple issues grow into headaches, restless nights, or poor school performance. It shouldn’t take a crisis.
Sodium ferrous citrate can help those who struggle with other supplements, but problems remain. Many people buy whatever the pharmacy staff recommends, without a blood test or doctor’s input. Folks with kidney trouble or chronic illnesses don’t always realize how iron can affect other parts of their body. Iron pills, in any form, can cause trouble if folks take too much. Sometimes new supplements don’t fix the root cause, like stomach ulcers or poor diets, so they just mask bigger problems.
Many adults mix up iron-rich foods with easy solutions. A steak dinner or spinach salad now and then won’t solve long-term deficiency. Research from the World Health Organization says nearly two billion people worldwide deal with iron deficiency, yet accurate information remains tough to pin down. Often, busy clinics only offer a quick fix, rather than explaining that supplements like sodium ferrous citrate work best alongside balanced meals and medical follow-up.
Pharmacists and doctors do their best, but patients move quickly. In low-income areas, quality supplements run scarce, so people rely on store brands that might not list sodium ferrous citrate amounts clearly. Some get frustrated by slow results, not realizing the body takes weeks or months to rebuild iron stores.
Solving iron deficiency usually means more than just swallowing pills. Clear labeling, training for pharmacists, and tougher regulations for over-the-counter supplements can save lives. GPs who take time to listen and recommend blood tests create better outcomes. If schools and community clinics hand out easy-to-read pamphlets with practical advice, more people start asking the right questions. Regulators who require companies to list accurate amounts of sodium ferrous citrate do everyone a favor.
Small changes in daily routines, like pairing iron pills with foods that help absorption and skipping those that block it, offer smart, science-backed ways to turn short-term patchwork into lasting health. Sodium ferrous citrate serves best as one tool among many in tackling iron deficiency—never a fix-all.
Iron deficiency can sap energy, bring daily fatigue, trigger headaches, and cause more serious problems over time. More than a billion people grapple with anemia every year, according to World Health Organization estimates. For anyone caught in that struggle, doctors sometimes suggest sodium ferrous citrate, a form of iron that the gut can absorb without as many stomach problems. The real draw for many people lies in the lower risk of common side effects like nausea and constipation.
Doctors usually suggest taking sodium ferrous citrate on an empty stomach since the body absorbs iron much better that way. A glass of water and nothing else about one hour before food, or two hours after a meal, makes a big difference. Dairy, tea, coffee, and some grains can block iron absorption. Many folks rush to take their supplements alongside breakfast, but it pays off to wait a bit before that morning coffee or bowl of cereal.
Some people feel queasy with any iron pill, empty stomach or not. If that happens, it’s usually safer to eat a small amount—not a whole meal, but maybe a cracker or piece of toast. Sticking to bland foods lets the supplement work while sidestepping harsh reactions.
Taking iron with antacids or calcium-rich foods makes it less likely to do its job. Multivitamin time and iron pill time are best kept apart by at least two hours. Many antibiotics and medicines for thyroid issues don’t mix well with iron, so a chat with a pharmacist or doctor goes a long way. This isn’t about being fearful, just practical—lots of us juggle busy medication schedules.
The exact amount and frequency depend on blood test results, age, sex, and medical history. Doctors often check bloodwork every couple of months. The results show if the iron is helping or if anything needs tweaking. Some iron-deficiency cases call for daily use, others ask for every other day, since the body sometimes absorbs it better with a break in between. No one iron schedule fits all. Keeping an honest log of supplements helps avoid missing doses or accidentally doubling up.
Many people get discouraged by the metallic taste or change in stool color. These things sound alarming but aren’t usually dangerous. Consistency beats perfection—a few missed doses won’t undo progress, but skipping for a week or bouncing between products slows recovery. Tracking for side effects, like stomach pain or rash, and reporting anything unusual to a healthcare professional can clear up confusion.
Pairing pills with vitamin C, such as orange juice, has been shown to boost iron absorption. Staying hydrated helps, too, since dehydration can make side effects worse. It can take weeks—or months—for low iron levels to fully recover, even with daily use.
Partnering with a healthcare provider to set up clear routines encourages steady progress. Family or phone reminders help with sticking to the schedule. Communities with high rates of anemia see better results when clinics provide clear education about iron intake, what foods to avoid around dose time, and reasons behind those recommendations. Good habits plus support make it less daunting in the long run.
A lot of doctors write up sodium ferrous citrate for folks dealing with iron deficiency. You hear “iron supplement,” you picture stronger muscles and extra zip in your step. Still, nothing in life comes without trade-offs, including pills to pump up your blood. I’ve watched family members swallow iron tablets after every meal, only to drop the habit because of unexpected stomach misery. Reading patient forums, you’ll find the same complaints—so let’s cut through the fine print and talk straight about what this compound can do inside the body, for better or worse.
The most frequent gripes about sodium ferrous citrate sound familiar: nausea, constipation, diarrhea, and an upset stomach. Sometimes pills leave a metallic taste behind. Kids, older folks, or people with sensitive bellies seem to notice these effects faster than others. Studies from Japanese hospitals, where sodium ferrous citrate gets prescribed often, back this up—over a third of patients in some reports mention some sort of stomach trouble.
Doctors often encourage patients to take iron after food, hoping food will cushion the blow. It doesn’t always save you from discomfort. I’ve seen folks try ginger tea and yogurt to soothe their digestion afterward. These small steps sometimes help, but no trick works every time.
Some side effects reveal themselves in weird ways. Stools turn darker—nearly black. That look can scare some people, but this color change signals your gut is handling the iron, not a sign of internal bleeding. Still, it’s worth a quick Google search or chat with your pharmacist if it catches you off guard.
Other reactions show up less often, but nobody’s immune. Rash, itchiness, or swelling sometimes point to allergies—those are signals to seek help fast. Dizziness or headaches crop up for a few people, though not as often as stomach issues. People with chronic health conditions—like kidney problems—face bigger risks. Iron can build up and trigger toxicity, leading to fatigue, joint pain, or even organ trouble. Too much iron gets dangerous a lot faster in young children, so homes with both supplements and toddlers require care.
Careful dosing matters most. A doctor’s advice should count more than a friend’s experience or tips from a supplement aisle. Lab work tells the truth about iron levels, not a hunch or guess. Tracking side effects in a simple notepad helps; I’ve watched friends swap brands or switch to a liquid form under a doctor’s eye, sometimes easing problems.
The U.S. National Institutes of Health, Mayo Clinic, and Japan’s PMDA have all put out clear guidance on how to use iron safely. They recommend regular follow-ups, plenty of fluids, and a close watch for red flags. If unpleasant symptoms linger, it’s not a sign to “tough it out” but a reason to check in with a medical professional who can fine-tune the plan.
No iron supplement fixes all the causes of tiredness or weakness. Doctors often remind patients to focus on food choices—spinach, beans, meat—before relying on tablets alone. Healthy eating usually spares people the worst of the side effects, or at least reduces how much extra iron they need. In my own family’s case, gradual, informed changes made a bigger difference than anything that came in a bottle.
Plenty of doctors talk about iron supplements during pregnancy. As someone who spent hours reading up on pregnancy-safe foods and supplements, I realized that not all iron sources are equal. Sodium ferrous citrate pops up often, especially in prenatal vitamins. It’s an iron salt, meant to help boost low iron levels – a common problem as the body works overtime to grow a baby and support the placenta.
Many pregnant women develop mild anemia because of increased blood volume and iron needs. Without enough iron, fatigue sets in, along with higher risks of preterm birth and low birth weight. I still remember how energy levels dropped until the doctor caught my low hemoglobin on a routine test, then brought up supplements.
Pregnancy diets often miss the mark. Even a menu filled with spinach, beans, and lean meat can fall short of the 27mg daily iron target suggested by the National Institutes of Health for pregnant women. That’s where sodium ferrous citrate enters, promising better absorption and fewer stomach problems compared to older iron pills like ferrous sulfate.
Doctors prescribe sodium ferrous citrate worldwide, counted on for decades. Most studies point out that it gets absorbed well and doesn’t usually upset the stomach as much as other forms. Less nausea and constipation make a huge difference, especially in the first trimester, when many struggle to keep food down.
The US Food and Drug Administration classifies iron supplements for pregnancy as likely safe when used correctly. In Japan, sodium ferrous citrate is a go-to prescription. Indian guidelines also put it in their maternal care programs. Research in medical journals confirms safety in recommended doses. Iron overdose, though, causes real problems: stomach pain, vomiting, and even organ issues if too much stacks up in the body. No one should self-prescribe or double up just because of tiredness.
Any supplement comes with potential side effects. Sodium ferrous citrate can still cause nausea, constipation, dark stools, and stomach discomfort. Taking it with vitamin C (like orange juice) helps iron absorption, but taking it with antacids or caffeine cuts absorption instead. Too much iron builds up slowly and rarely causes trouble for short periods, but long-term heavy use can lead to iron overload. Women with blood disorders like thalassemia or hemochromatosis need extra caution and constant supervision.
Doctors want to see bloodwork before giving out iron prescriptions. A balanced approach starts with diet: lean meats, leafy greens, lentils, and iron-fortified grains. Most doctors check ferritin and hemoglobin during routine prenatal visits, then suggest starting supplements if numbers dip too low.
Take sodium ferrous citrate only as directed, usually with water or juice, and avoid milk or calcium-rich foods around the same time. Side effects tend to show up in the first weeks, but letting your doctor know about any changes gives the best chance for a healthy pregnancy.
Trust in sodium ferrous citrate comes from real-world experience and solid research. Doctors back its safety when the dose fits medical needs. Open conversation with care teams and paying attention to signals from your body makes a difference. No supplement replaces a varied diet, but the right iron support often keeps both mother and baby strong throughout pregnancy.
Taking care of iron levels matters for anyone dealing with iron deficiency anemia, and sodium ferrous citrate often gets prescribed for this reason. This iron supplement absorbs easily and works well for many. On the surface, popping a pill or two per doctor’s orders doesn’t seem complicated. Truth is, mixing it with other medicines, or even vitamins and foods, may change how it works—or how well your other meds work for you.
Doctors and pharmacists often bring up drug interactions for good reason. Left unchecked, even something as basic as an iron tablet, especially sodium ferrous citrate, causes some surprises. Think about antacids for digestive issues. Products with calcium, magnesium, or aluminum interfere with iron absorption. Swallowing that antacid after a spicy meal and then taking sodium ferrous citrate a few minutes later might mean your body gets less iron than intended.
Antibiotics, especially those like tetracycline and ciprofloxacin, also come up in conversations about drug interactions. Iron binds with these antibiotics in the digestive tract, lowering the amount your body can absorb. This means the infection you’re treating might stick around longer, and the anemia might persist, too. Always a good idea to separate these meds by a couple of hours, giving each a fair chance to do its job properly.
Thyroid medications, including levothyroxine, tell a similar story. Iron can attach to this medication and reduce its power, leaving folks still feeling tired and foggy. A few simple timing tweaks—spreading out these meds—lets each medicine work the way it should.
It’s tempting to think only prescription pills matter, but vitamins and even some foods can take their toll on iron absorption. Vitamin C, found in citrus fruits or as supplements, boosts iron absorption. On the flip side, dairy and high-fiber foods like bran can make things tougher for iron tablets to work. Even some herbal teas change how the gut handles the iron.
People sometimes think vitamins and supplements are harmless, but they still need respect. Multivitamins often come loaded with calcium or zinc—both clash with the iron in sodium ferrous citrate. Taking them together can set you back if you’re trying to escape chronic fatigue or low hemoglobin.
I’ve seen family members juggle pill schedules, not always realizing how a daily glass of milk screws up iron absorption. It only took a straightforward chat with a pharmacist to learn what foods and drugs to keep apart. That one change brought up energy levels and cut down sick days. Proper timing and a list of medicines on hand at doctor's appointments help avoid pitfalls before they start.
No one expects you to remember every drug interaction. Pharmacists are there for a reason, and resources like medication checkers online help keep things clear. Simple steps—logging all supplements, asking questions, readjusting timing—stop issues before they derail progress. Instead of taking sodium ferrous citrate and hoping for the best, a bit of effort can turn that hope into steady improvement.
Iron deficiency can weigh anyone down, but mixing sodium ferrous citrate with the wrong meds only adds to the struggle. Keeping track, asking for advice, and reading labels go a long way toward making sure your treatment pays off.
| Names | |
| Preferred IUPAC name | Sodium 1-carboxyethoxy-1,2-dioxoethane-1,2,3-tricarboxylatoferrate(2-) |
| Other names |
Sodium iron(II) citrate Ferrous sodium citrate Sodium citrate, iron(II) complex Ferricitras natricus SFC |
| Pronunciation | /ˈsəʊdiəm ˈfɛr.əs ˈsɪtrət/ |
| Identifiers | |
| CAS Number | [141-01-5] |
| Beilstein Reference | 3576796 |
| ChEBI | CHEBI:9410 |
| ChEMBL | CHEMBL1201616 |
| ChemSpider | 21586195 |
| DrugBank | DB14045 |
| ECHA InfoCard | 054206KNZG |
| EC Number | 237-749-5 |
| Gmelin Reference | 86367 |
| KEGG | D02041 |
| MeSH | D04722 |
| PubChem CID | 10690 |
| RTECS number | DJ1430000 |
| UNII | YS4Y4ZZ64A |
| UN number | UN2811 |
| CompTox Dashboard (EPA) | DJ9T2V9T4W |
| Properties | |
| Chemical formula | C6H6FeNaO7 |
| Molar mass | 446.14 g/mol |
| Appearance | A reddish-brown powder |
| Odor | Odorless |
| Density | 1.2 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -3.8 |
| Acidity (pKa) | 3.5 |
| Basicity (pKb) | pKb ≈ 3.0 |
| Magnetic susceptibility (χ) | Paramagnetic |
| Viscosity | Viscous liquid |
| Dipole moment | 0.00 D |
| Pharmacology | |
| ATC code | B03AA10 |
| Hazards | |
| Main hazards | May cause irritation to skin, eyes, and respiratory tract. Harmful if swallowed. |
| GHS labelling | GHS07, GHS08 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | Harmful if swallowed. Causes serious eye irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Avoid contact with eyes, skin, and clothing. Wash thoroughly after handling. Use with adequate ventilation. Do not ingest or inhale. |
| Lethal dose or concentration | LD50 (oral, rat): 680 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 1,760 mg/kg |
| PEL (Permissible) | Not established |
| REL (Recommended) | 50 mg (as iron) daily |
| Related compounds | |
| Related compounds |
Iron(II) fumarate Iron(II) gluconate Iron(II) sulfate Ferric citrate Sodium citrate Ferrous ascorbate |