Sodium lactate has history stretching back further than many realize. Chemists knew lactic acid from sour milk long before the twentieth century. The salt, though, gained traction with food preservation and medicine during the early and mid-1900s. Demand for reliable food preservation and safer medical solutions drove commercial synthesis. Factories scaled up, pharmacists bottled it, and suddenly, sodium lactate became a pantry staple and a familiar presence in clinics and operating rooms. Regulations kicked in across Europe, the US, and Asia, setting standards and sparking further development. The story grew from kitchens to laboratories, bringing together agricultural roots and modern chemistry.
Sodium lactate turns up in solid and liquid forms, depending on what folks need. You spot it in everything from food-grade syrups to pharmaceutical-grade solutions sitting on hospital carts. Some use it to keep meat juicy, others to replenish electrolytes in IV drips. Production’s pretty reliable these days, with factories churning out barrels for different industries. Whether labeled for food, medical, or personal care, the main idea stays the same—get a safe, shelf-stable, slightly salty solution ready for action.
Sodium lactate doesn’t try to hide. It’s colorless when dissolved in water, giving off only a faint sweet or salty odor. The taste falls between mildly salty and a little bit alkaline. As a salt of lactic acid, it handles moisture like a pro, soaking up water from the air if left uncovered. Its pH sticks on the slightly basic side, usually landing somewhere between 6.5 and 8 in solution. That’s helpful since the chemical stability means food won’t spoil so quickly. Its solubility and melting point make it easy to use, blend, and store in warehouses or hospitals.
Suppliers and regulators set clear lines for what counts as high-quality sodium lactate. Industrial batches post specs—purity above 98%, minimal lead, arsenic, or heavy metallic impurities. Liquid forms come described by molarity or percent weight. Labels need to declare the source (like corn or beet), grades (such as USP, EP, or food), and any allowed additives, according to strict international rules. For food, look for E325. In pharmacy, watch the grade and batch traceability. Products listing sodium lactate come with full compliance paperwork, especially for pharma and injection use.
Manufacturers start with fermenting sugars, usually from corn or beets, with lactic-acid bacteria doing the heavy lifting. As the bacteria chow down on the sugar, lactic acid forms. Neutralization comes next, often with sodium hydroxide, producing sodium lactate plus water. The product gets purified through filtration and sometimes ion-exchange or distillation. Careful technicians adjust the concentration and pH, test stability, and direct the final solution to bottling, blending, or further processing, depending on its end use.
Sodium lactate steps up as a mild base, reacting with acids to regenerate lactic acid. It survives most household chemistry. With strong oxidizers or high heat, it breaks down and gives off water and carbon dioxide. Scientists in food tech and pharma keep tweaking it—attaching other groups or turning it into esters to fit new preservation tasks. It helps chelate metals or buffer other salts, giving products a longer shelf life or gentler taste.
Shoppers and lab techs might spot sodium lactate on labels under names like E325, lactic acid sodium salt, or the less catchy “sodium 2-hydroxypropionate.” In different languages and industries, these synonyms stick to local customs or labeling laws. Its various forms all circle back to the same compound, just dressed up in different packaging or concentrations.
Regulators like the FDA, EFSA, and WHO list sodium lactate as generally recognized as safe (GRAS) in food. Still, standards sit in place for handling, transport, and quality. Storage asks for tightly sealed containers, out of reach of moisture and away from strong oxidizers. In hospitals, only qualified staff prep and inject sodium lactate solutions to avoid dosage mistakes. Each facility draws up safety protocols for spills, skin contact, or accidental ingestion. Like every food additive, the supply chain faces audits and frequent checks for contaminants or mislabeling.
Supermarkets, restaurants, and hospitals all lean on sodium lactate, sometimes without realizing it. Meat and seafood stall owners swear by it for holding in moisture and boosting shelf life. Bakers use it to raise pH and give bread a better crust. In IV bags, it corrects acid-base imbalances and keeps patients hydrated. Soap and lotion makers value it for its humectant qualities, drawing moisture to the skin while keeping products smooth. Some folks in the chemical industry mix it into cleaners and descaling agents for its buffering kick.
The last decade brought new eyes to sodium lactate. Sustainability drives research into greener fermentation processes, while the food industry looks at minimizing sodium content without sacrificing performance. Bioplastics research taps sodium lactate for new blends of biodegradable materials. Meanwhile, pharmaceutical teams study its role as a carrier for tricky drugs, exploring the limits of shelf stability. Journals keep churning out studies, each promising a small leap in either efficiency, safety, or broader application.
Animal studies and long-term exposure tracking show sodium lactate sitting low on the danger scale. Researchers monitor its effects at both dietary and medical doses, always on the lookout for hidden risks. Mild irritation can hit if splashed on sensitive skin, and overdosing in IV form sometimes brings on shifts in sodium or pH balance, but regulated use stays well below dangerous levels. Health agencies keep updating tables and guidelines based on medical data from hospitals and manufacturing plants.
With changing diets, stricter food regulations, and ongoing pressure to trim sodium in processed foods, sodium lactate’s future looks busy. The food industry expects more demand for alternatives to traditional preservatives. Medicine continues to lean on it for both routine and emergency fluids. Bioplastic startups might pick up steam, increasingly using sodium lactate as a safer, biodegradable ingredient. Researchers see untapped potential in modifying it for specialized medical formulations and long-life foods. As global markets shift toward sustainability and better health, sodium lactate seems set to keep earning its keep in pantries, labs, and factories for a long time yet.
Sodium lactate tends to show up where most folks wouldn’t think to look. The clear liquid often acts as a quiet helper in food, medicine, cosmetics, and even homemade soap. I noticed its name on a deli meat label once and decided to dig into what makes it so popular—and worth noticing.
Anyone who’s tried to keep leftovers from spoiling too fast knows how tricky that can be. Food companies learned to use sodium lactate to stretch shelf life, especially in meats. This isn’t about cutting corners; it’s one way to keep harmful bacteria, like Listeria, from gaining a foothold. Its salty taste helps bring out flavors, making turkey slices or roast beef a bit more savory.
Chefs and food processors see it as a tool, not just a preservative. Sodium lactate keeps meats juicy through the week. It slows down water loss, so store-bought rotisserie chicken holds up longer in the fridge. That means shoppers don't toss so much food, and stores worry less about waste.
Hospitals rely on sodium lactate in IV drips, especially for patients who need fluids quickly. After climbing a hill and running out of breath, I understand the role rehydration plays. Emergency rooms use sodium lactate to balance acids in the body when someone’s sick or dehydrated.
Some doctors also use it to treat certain types of acidosis, where the blood turns too acidic. It helps get things back to normal, often when there’s no time to waste. Medical technicians pay close attention to how much gets used, because too much can be just as bad as too little.
Whole aisles of fancy hand soaps use sodium lactate to attract moisture. Whenever I’ve tried making soap at home, adding a little of it makes bars harder and longer-lasting. It draws in water from the air, so hands don’t dry out after each wash. Cosmetics makers turn to sodium lactate in creams aimed at keeping skin supple, especially through cold winters.
One thing to watch: Sensitive skin might react if there’s too much. Choosing well-formulated lotions makes a difference, so reading labels goes a long way.
Like any additive, sodium lactate brings up debates about how much is safe. Regulatory bodies like the FDA approve its use, but some consumers worry about processed foods in general. I believe clearer labeling and honest communication help people make decisions that fit their health goals. Too much salt isn’t good, and sodium lactate contains sodium, so folks with high blood pressure need to pay extra attention.
On the food waste front, sodium lactate supports efforts to keep perishable foods safe a bit longer. It won’t solve the problem itself. Smarter inventory systems in stores and better storage advice at home should work alongside safe preservatives like this one.
Most don’t give much thought to what keeps everyday products safer or more comfortable. Sodium lactate proves that little helpers often play a big part, lurking quietly in ingredient lists. Whether at the dinner table, in a hospital room, or beside a bathroom sink, this common additive does some behind-the-scenes heavy lifting. Maybe that’s one ingredient label worth a second look.
Most folks have taken a spin through the ingredient list on a tube of hand lotion and spotted words like sodium lactate. Sometimes this stuff sounds mysterious or just plain chemical, and of course, no one wants to rub something sketchy into their skin. My first impression wasn’t much different. But once you dig in, sodium lactate turns out to be a real workhorse behind the scenes in plenty of skincare routines.
Sodium lactate comes from lactic acid, which itself pops up pretty much everywhere: in milk, in fermented foods, and even in our own skin. Think of it as a salt version of lactic acid, helping keep things balanced. In skincare, it pulls double duty—it helps skin hold onto water, so you get less flaky dryness, and it also smooths out rough patches. It's common in creams and serums that promise a “hydrating” effect.
Most safety talk circles back to one point: scientists and dermatologists have checked out sodium lactate in cosmetics for years. Several big organizations, including the US Food and Drug Administration and the Cosmetic Ingredient Review Expert Panel, have done their research. Sodium lactate rarely triggers allergic reactions. In common moisturizers, low concentrations keep things gentle. The European Commission greenlights sodium lactate in rinse-off and stay-on products, as long as it sticks to safe concentrations.
If you dig deeper, you see why sodium lactate gets the thumbs-up. Our own bodies make lactic acid as we break down food. Sodium lactate just borrows from a process that happens naturally, winding up as a humectant that doesn’t throw the skin’s ecosystem out of whack. Anyone fighting winter dryness, itchiness, or even mild eczema might find these hydrating products bring real relief without the baggage of synthetic additives that can irritate sensitive skin. I’ve watched plenty of friends with stubbornly dry shins or elbows finally find something that works—turns out, sodium lactate’s usually in the mix.
Not every ingredient plays nice with everyone. Fair point. For those with ultra-reactive skin, even everyday hydrators can cause redness or stinging, especially at higher strengths. Sodium lactate mostly flies under the radar, but concentrations over 10% can stir up tingling or minor irritation. Stick to reputable brands, look for products that actually list their percentage, and try spot testing before slathering it everywhere. If you do feel burning or see redness, rinse off, and dial back. It's the same practical sense you'd use for anything new.
Sodium lactate doesn’t work alone. In a well-made moisturizer, you’ll see it paired with other gentle ingredients like glycerin, ceramides, and sometimes oils. This mix supports the skin barrier, locking in moisture but letting your skin breathe naturally. There’s no reason to demonize sodium lactate just because it sounds a little technical. Plenty of studies back its safety. The real trick lies in the recipe—too much of any one thing can mess with balance, whether it’s a natural acid or an essential oil.
As someone who’s tried a drawerful of face creams and body lotions, I’ve learned that transparency pays off. If labels clearly list how much sodium lactate they include, customers can make safer choices. Education also matters; stores and brands could do a better job helping folks understand why certain ingredients land in their favorite products. When people trust what's inside the bottle, they use it the right way and dodge unnecessary worries about safety.
Walk into a grocery store, pick up a loaf of bread, a bottle of moisturizer, or a packet of preserved meat, and one ingredient shows up more than you’d expect: sodium lactate. The debate about its “naturalness” has sparked plenty of online chatter, especially as people grow more aware of labels and what goes into their bodies. But what really separates something natural from synthetic on that ingredient line?
Sodium lactate starts out with sugar or starch—anything from corn or sugar beets can fuel the process. Add bacteria, and fermentation gets going, just like when making yogurt, cheese, or sourdough. This process creates lactic acid, a substance that I always thought of as simply a byproduct in sour milk. In food production, companies then blend lactic acid with a simple sodium compound (like baking soda) and, voilà, sodium lactate. The journey doesn’t end on a farm, though. After fermentation, factories step in. Purification, concentration, and mixing make the finished salt that shows up in ingredient lists.
With both steps—natural fermentation and lab refining—the question about being “natural” gets complicated. Fermentation is about as straightforward and old school as it gets. Turning lactic acid into sodium lactate with chemical reagents gives things a more industrial edge. It’s not pulled from the earth, nor does it grow on trees, but the processes don’t introduce any strange or unsafe chemicals either. That’s more than I can say for some long-named additives.
People care about sodium lactate’s source because the word “synthetic” gets bad press. Plenty of folks see “natural” on packaging and trust it. Some worry about allergy triggers or want things as close to farm-fresh as possible. But what matters for health goes beyond labels. The FDA recognizes sodium lactate as safe. The European Food Safety Authority has done the same. I’ve never seen a recall or a panic tied to the ingredient itself—usually more fuss comes from improper storage or other additives in a finished food.
What I have noticed in my own kitchen is that sodium lactate does what it says on the tin. It pulls water to the food, keeps things moist, and helps prevent spoilage. In tough climates or long transport, a preservative like this cuts down on food waste. In hospitals, they count on it to hydrate patients' bodies. These are real everyday benefits that impact health more than the label debate.
Instead of dividing ingredients into “good” and “bad” boxes, I’d rather see more honesty on packaging. There’s no magic in saying something’s “natural” when that mostly depends on loose definitions and creative marketing. Real trust forms when brands show how things are made, why they pick certain ingredients, and what possible side effects could show up for sensitive groups.
Anyone with allergies, special diets, or just a curious palate should read labels, look for certifications if that matters to them, and, most importantly, dive into the science themselves. Producers could help by giving straight answers about processing steps, explaining the source, and avoiding marketing jargon. Sodium lactate isn’t a villain or a saint—just one more example of how modern food walks a fine line between farm and laboratory.
Sodium lactate turns up in more foods than people might guess. This salty liquid comes from the fermentation of sugars found in things like corn or beets. Folks have seen it on ingredient labels, maybe shrugged, and wondered what it does. The truth is sodium lactate brings a handful of real uses to the food table. Chefs rely on it for more than just extending shelf life or boosting flavor. It has ties to safety, taste, and even texture.
Growing up in a family that valued packed lunches, I learned firsthand how delicate leftovers can be, especially during summer. Sodium lactate slows the growth of bad bacteria, which means meats and ready-to-eat meals stay safer for longer. Instead of watching fresh deli turkey spoil in two days, lunch stays good through the week. For people worried about foodborne illness, this small ingredient plays its part. Meat processors, for instance, add it to ham or chicken to keep listeria at bay. Nobody wants a recall traced back to their kitchen, and that kind of risk isn’t just hypothetical; the CDC tracks outbreaks every year, and sodium lactate sits among the tools that cut those numbers down.
Plenty of folks appreciate a roast that holds onto its juices or a packaged sauce that keeps tasting fresh. Sodium lactate pulls water into the meat, letting it cook up a little juicier. At home, I noticed sausages stay plumper when this ingredient is in the mix. Bread makers also sneak it into dough, since a steady moisture level makes for a softer slice, even after a few days on the shelf. These aren’t just manufacturing tricks—home cooks borrow the technique to reduce waste and score better results from weeknight meals.
Skepticism about food additives isn’t new. Walking supermarket aisles, I hear people mutter about “chemicals” on ingredient panels. Sodium lactate sometimes winds up on those lists of suspicious-sounding substances. It’s tough to change minds when jargon gets in the way, but sodium lactate comes from plain fermentation, and the body deals with it like any other salt. Researchers haven’t linked it to allergic reactions in healthy folks or found evidence of danger in the amounts used in food.
People asking about natural substitutes have options. Lemon juice, vinegar, and celery powder all offer ways to build a clean label, but those swaps cost more and can shift flavors or shelf life. Small food brands might not manage that extra expense. If customers push for ingredients they recognize, some processors will listen and experiment with new recipes. Home cooks can always skip processed meats and buy fresh when possible, too.
There’s always room for plain talk about what goes into food. Sodium lactate isn’t magic, and it isn’t a villain. At best, it keeps food safer and tastier for longer stretches, and for many families juggling busy lives, that means fewer rushed trips to the store or wasted leftovers. Watching trends and reading up on new studies helps everyone push for smarter choices without falling for marketing noise.
If you ever walked through a food lab or peeked into a soap maker’s workshop, sodium lactate probably sat somewhere on a shelf in a crisp bottle. People reach for it because it mixes easily, works well in preserving food, and adds that little bit of “slip” in personal care products. You’d think something this useful could stick around forever, but that’s not how it plays out.
Experience in food service and cosmetics teaches a person to check dates. For sodium lactate, those details come down to smart storage and packaging. Most manufacturers stamp shelf lives of around two years on unopened bottles. That figure looks comforting, but chemistry and real-world kitchens tell another side of the story.
Exposure to heat, humidity, and direct sunlight takes a toll on even the purest ingredients. Sodium lactate, in liquid form, draws moisture from the air. Over time, a tightly sealed bottle keeps out water and airborne particles, but once opened, that barrier only does so much. Instead of trusting blank dates, professionals sniff and check for cloudiness or sediment buildup before use. Changes in smell or texture often give the best clues.
At a commercial bakery I managed, we once had a run-in with a batch that sat too close to the oven racks—bad move. Warmer storage sped up the aging of sodium lactate, leading to an off-taste in our next loaf. Storing the bottle in a cool, dry place became rule number one. Some creative home crafters stick their sodium lactate in the refrigerator for longer life, but usually, anywhere consistently under 25°C works.
Tightly screwing the cap back after every use and staying away from metal scoops prevents contamination. Moisture and foreign material spark spoilage long before the “best by” date. High humidity, for example, can lead to crystallization—clumpy crystals at the base mean the ingredient isn’t doing its job inside your product.
Published research shows that temperature control can double the storage time for sodium lactate. The World Health Organization notes that improper handling can cut shelf life, causing organic breakdown and a bitter flavor. In artisanal skincare groups, failed batches point straight to old or poorly sealed sodium lactate.
Food manufacturers run regular quality checks with high-performance liquid chromatography; these labs routinely toss stock over twelve months old, especially if the seal broke repeatedly. Home users, watching pennies, often push products past those dates, but headache and wasted effort soon follow.
All sorts of people need better clarity when storing specialty ingredients. Greater use of tinted bottles or single-serve packets could curb degradation. Printed storage reminders on the bottle do more good than fine print buried in data sheets. Some producers roll out freshness indicator stickers, which change color if a product gets too warm. At home, a dry cupboard—the kind away from steamy stoves or sunlit windows—will do fine.
Ultimately, sodium lactate is no different than coffee or flour—how you treat it from day one decides how well it does its job. Respect expiration dates, store thoughtfully, and you’ll save both effort and cash in the long run.

| Names | |
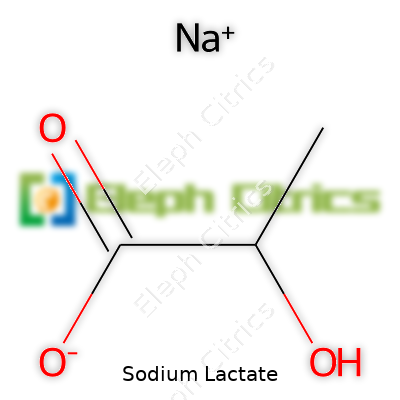
| Preferred IUPAC name | Sodium 2-hydroxypropanoate |
| Other names |
Lactic acid sodium salt Sodium 2-hydroxypropanoate E325 |
| Pronunciation | /ˌsəʊ.di.əm ˈlæk.teɪt/ |
| Identifiers | |
| CAS Number | 72-17-3 |
| Beilstein Reference | 3561401 |
| ChEBI | CHEBI:6636 |
| ChEMBL | CHEMBL1351 |
| ChemSpider | 54659 |
| DrugBank | DB09161 |
| ECHA InfoCard | 100.028.288 |
| EC Number | 200-018-0 |
| Gmelin Reference | 7930 |
| KEGG | C01781 |
| MeSH | D017325 |
| PubChem CID | 23663865 |
| RTECS number | OU8750000 |
| UNII | 8P9E0OZ36Q |
| UN number | UN2058 |
| Properties | |
| Chemical formula | C3H5NaO3 |
| Molar mass | 112.06 g/mol |
| Appearance | Colourless or almost colourless, clear, slightly viscous liquid |
| Odor | Odorless |
| Density | 1.33 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.8 |
| Acidity (pKa) | pKa 3.6 |
| Basicity (pKb) | Sodium Lactate has a pKb of approximately 12.8 |
| Magnetic susceptibility (χ) | -13.0×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.431 |
| Viscosity | Viscous liquid |
| Dipole moment | 1.82 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 150.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -730.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -1616.8 kJ/mol |
| Pharmacology | |
| ATC code | B05BB01 |
| Hazards | |
| Main hazards | May cause mild skin and eye irritation. |
| GHS labelling | Not a hazardous substance or mixture according to the Globally Harmonized System (GHS) |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing. If eye irritation persists: Get medical advice/attention. |
| Lethal dose or concentration | LD₅₀ (Oral, Rat): 2000 mg/kg |
| LD50 (median dose) | LD50 (oral, rat): 2000 mg/kg |
| NIOSH | WGK3 |
| PEL (Permissible) | 15 mg/m³ |
| REL (Recommended) | 2% |
| IDLH (Immediate danger) | No IDLH established |
| Related compounds | |
| Related compounds |
Lactic acid Potassium lactate Calcium lactate Magnesium lactate |