Strontium citrate traces its origins back to the early 19th century, a time marked by the growth of inorganic chemistry and new attention to both strontium and citric acid. Early chemists focused on understanding strontium’s presence in minerals and how to extract salts with practical value. By the late 1800s, researchers documented reactions between strontium compounds and organic acids, including citric acid, in an environment filled with experimentation but lacking today’s rigorous standards. It didn’t take long for medical circles to start paying attention, particularly as the implications of bone-health properties of strontium came into sharper focus. Throughout the 20th century, improvements in purification techniques and a better grip on molecular structures led to reliable methods of making strontium citrate, turning it from an experimental curiosity into a consistent product for both scientific research and commercial use. The historical arc of strontium citrate mirrors much of inorganic chemistry’s journey—from isolated discovery through careful validation, finally reaching modern systems that prize traceability and efficacy.
Strontium citrate appears in the form of a fine, white powder, sometimes with a granular texture, and is now used in both nutritional supplements and laboratory supply chains. It is a salt brought together by strontium ions and citrate ions, cemented by improvements in control over purity and batch consistency over decades of development. Many manufacturers sell it as a dietary supplement, usually for bone health, stating specific amounts per capsule and verifying strontium content using standardized laboratory assays. Commercial grades show little difference to the naked eye, though certificates of analysis separate high-quality lots from lower-grade stock. Researchers value the compound for its solubility and neutral taste in solution, which gives it an edge over other strontium salts that might otherwise introduce metallic or bitter notes. Product packaging often lists not just the strontium content, but also rigorous quality control information, batch traceability, and warnings required by law.
Freshly prepared strontium citrate forms a white-to-off-white powder, with low moisture content and little inclination to cake or clump under normal storage. The compound does not emit a discernible odor and does not react with air at room temperature. Water solubility stands at about three grams per liter at ambient conditions, making it much more approachable than strontium carbonate or sulfate, which resist dissolution. Structurally, this salt embodies a typical ionic signature but gains a distinctive coordination pattern from citrate’s three carboxylate groups, which bind to strontium ions in solution and set up a stable molecular network. The melting point exceeds 200°C, though decomposition occurs before true melting in most real-world settings. pH of a saturated aqueous solution tends toward neutral or slightly acidic, reflecting the buffering effect of citrate.
Suppliers mark out technical specifications based on purity, typically demanding at least 99% assay by dry basis for laboratory and nutritional uses. Certificates of analysis confirm not just total strontium content, but also related anion and cation impurities (like calcium, sodium, barium, and magnesium) that could impact biological activity or product performance. Lead and heavy metal limits fall well below one part per million, matching regulatory expectations and consumer trust. Moisture often remains under 2%, as higher levels can promote clumping and degradation. Particle size distribution matters—most commercial powders pass a 100-mesh sieve, blending smoothly into tablets, capsules, or water-based solutions. Safe use depends on clear labels, stating total weight, strontium content, storage instructions, batch numbers, and expiration dates—each detail satisfying compliance rules in North America, Europe, and other strict regions. Warning language about use by children, pregnant individuals, and people with kidney disease signals increased scrutiny under evolving food and supplement safety laws.
Making strontium citrate involves a straightforward double displacement reaction. Starting with either strontium chloride or strontium carbonate, manufacturers blend in a solution of citric acid under gentle heat and continuous stirring. Strontium ions swap places with sodium, calcium, or chloride ions, falling out of solution as strontium citrate precipitate. Careful pH control avoids forming strontium oxalate, an insoluble contaminant. After filtration, repeated washing removes unreacted reagents and soluble byproducts. Drying happens at low temperatures under vacuum or in a clean oven to guard against decomposition and clumping. Automated systems increasingly monitor each step, from weighing through granulation, to support reproducibility. Operators sample each batch for purity and residual solvent content, often relying on titration, atomic absorption, or chromatographic techniques for confirmation. Final material undergoes a last pass through a fine mesh to assure even texture, then heads to packaging rooms built for minimal contamination and clear traceability.
In laboratory settings, strontium citrate shows stability against mild acids and bases, yet reacts with strong mineral acids to release citrate and form strontium chloride or nitrate solutions. Citric acid’s three carboxylic groups gear the molecule up for complexation chemistry, which draws in researchers studying metal ion chelation or pH-responsive drug delivery. Direct reactions with oxidizing agents yield byproducts like oxalates, acetates, and carbonates, though these transformations require deliberate effort or harsh conditions rarely met in supplement manufacturing. Some research teams experiment with doping strontium citrate with trace elements—or transforming it into strontium-substituted hydroxyapatite—to chase new therapeutic and imaging applications in biomedicine. Understanding these reactivity limits keeps production safe and product quality top-notch.
Beyond “strontium citrate,” you’ll spot the compound referenced by systematic names such as tristrontium dicitrate, or chemically as “strontium (II) citrate.” Supplement companies list both the salt form and total strontium per serving, with some products labeled “bone support complex” or similar marketing language. Older medical literature occasionally uses “citric acid, strontium salt” or “strontium (2+) citrate.” CAS Registry entries vary depending on hydration state (anhydrous or trihydrate), though commercial lines typically note “strontium citrate” plain and simple, to minimize confusion. No matter the name, regulatory filings cross-reference molecular formula and structure to enforce clear standards from batch to batch. Both US Pharmacopeia listings and European monographs detail common synonyms, tightening industry-wide recognition.
Operating standards for strontium citrate demand strict hygiene and cross-contamination prevention, reflecting lessons learned from earlier incidents involving heavy metal contamination in supplements. Facilities clean equipment between runs, document every material entry, and follow Hazard Analysis and Critical Control Points (HACCP) strategies. Material Safety Data Sheets flag mild irritant risks on inhalation or skin contact, though acute toxicity remains low unless someone ingests large quantities. Intake recommendations in supplements cap strontium exposure at a few hundred milligrams per day, far below hazard levels noted in animal studies. Occupational health teams install dust control and effective ventilation to protect staff handling large volumes. Regulatory oversight now includes regular on-site inspections, review of batch retention samples, and recall capability in the event of contamination. Clear labeling—reflecting food law, pharmacopoeia rules, and border inspection practices—adds another layer of safety, especially for end-users who require guidance about drug-drug interactions or underlying medical conditions that complicate strontium metabolism.
Health supplement markets take the lead, pitching strontium citrate for bone density support, particularly among older adults and people at risk for osteoporosis. The compound’s ionic radius closely matches that of calcium, nudging new bone mineralization in preclinical studies. Dental research labs look at the compound for restorative materials that encourage remineralization while resisting bacterial colonization. Strontium’s unique neutron-absorption profile also lands it in some niche nuclear and analytical chemistry applications. Academic teams reach for high-purity strontium salts like citrate for isotope labeling and Raman spectroscopic studies, especially if the samples serve as standards for environmental trace analysis. Veterinary researchers sometimes test strontium citrate in supplements for skeletal growth in certain animals. Broader technical interest circles around its chelating ability and use as a reference compound in metal-ion mobility experiments. Commercially, food technology, beverage fortification, and specialty ceramics manufacturers investigate new ways to blend small amounts without changing organoleptic or processing properties, though health-related applications still dominate.
Strontium citrate keeps drawing research dollars and scientific curiosity. Universities and contract research organizations design new trials to pinpoint not just population benefits for bone health, but also optimal dosing, metabolism, and long-term outcomes tied to supplemental use. Analytical method development has advanced, with atomic absorption spectrometry and ion chromatography now standard for quantifying both strontium and residual citric acid, resolving questions about batch-to-batch consistency. Material scientists dig into how citrate ligands change strontium ion migration through biological matrices, hoping to unlock new insights for both medicine and energy storage. Small biotech startups race to combine strontium citrate with other micronutrients, not just for human supplements but for applications in animal wellness and specialty feed. Clinical research moves slowly, complicated by the need for large sample sizes and the delicate balance of risks and benefits, though early trials continue to spark international interest. Research councils in the EU and Asia keep grants flowing for both preclinical and clinical arms of strontium research thanks to demographic trends toward aging populations.
Toxicologists learned early that natural strontium differs sharply from its radioactive siblings, and today’s focus has shifted toward understanding the chronically safe window for supplemental strontium citrate. Rodent studies show that at high intake, strontium can impair bone mineralization, especially in the context of immature or vitamin D-deficient animals, though such intake exceeds any practical dietary exposure. Genetic toxicity assays return negative, ruling out mutagenic risk under standard test regimes. The biggest concern lies in individuals with impaired kidney function, since strontium—unlike calcium—clears less efficiently, potentially building up in bone over long time frames. Current guidance from regulatory agencies highlights the need for better data on rare side effects, possible impacts on heart rhythm, and how strontium may interact with absorption pathways for other alkaline earth metals. Intestinal absorption rates hover around 25-30% for most adults, leaving the lion’s share to pass through without uptake. No acute toxicity or life-threatening response emerges under normal supplementation, but the watchful eye of food and drug agencies remains firmly in place.
Interest in strontium citrate shows no sign of cooling, as global shifts push more focus onto bone health and mineral supplementation. Ongoing research points toward targeted therapies, including new forms of strontium salts blended with vitamin K2, D3, or other micronutrients poised to deliver additive or synergistic benefits. Advances in nano-formulation could open new delivery channels, not just for oral supplements but potentially for injectable or topical products aimed at localized bone repair. Population aging in both developed and less-industrialized countries keeps demand rising, with policy frameworks pushing both research and consumer education. Trends in personalized nutrition and digital health monitoring may further expand strontium citrate’s reach, as tailored plans incorporate real-time mineral intake recommendations. Regulatory harmonization, stuck in piecemeal national approaches, looks primed for change as international trade and e-commerce force tighter standards and transparency. At the lab bench, strontium citrate acts as a springboard for broader research into rare earth and alkaline earth metal metabolism, keeping the door open for unexpected innovations in both medicine and materials science.
Walking into a supplement store, you’ll spot bottles promising better bones and a longer life. Most people think of calcium or maybe vitamin D when talking bone health, but strontium citrate has been climbing the shelves, too. It’s not as buzzy as the others, but those who look past the flashy labels sometimes find exactly what their bodies crave. Strontium, a trace element, shows up in both soil and veggies—so there’s a good chance you’ve already eaten some without knowing. The version called strontium citrate brings this mineral into a form that dissolves easily and gets absorbed by the digestive system.
Worry about breaking a bone only starts after a close call, or maybe you’ve seen an older parent shrink a little bit and start to shuffle more carefully. Studies show that strontium citrate may help maintain bone density, especially for people with a family history of osteoporosis. Two big ways our bones get weaker: less new tissue being built, and too much old tissue getting cleared out. Research led by European scientists points to strontium’s ability to support stronger bones by slowing breakdown and nudging the body to form more new bone. Busque and associates found postmenopausal women who used strontium supplements over a long period had fewer dangerous drops in spine and hip bone densities.
It’s one thing to read a data table, it’s another to watch a grandparent stand up easier in the morning. A close neighbor shared how her specialist recommended strontium citrate after she battled bone thinning in her 60s. After a year, her reports showed measurable improvement. She still takes calcium, and doesn’t skip her morning walk, but she credits strontium citrate for letting her keep up with her gardening. Researchers at McGill University observed similar outcomes, where consistent strontium citrate intake coupled with exercise brought positive changes for folks at risk of fractures. Marketing hype can sound the same for every supplement, but the stories from local people hit differently.
Like all things, more doesn’t always mean better. The body only needs trace amounts—overdoing strontium could cause issues, especially if kidneys aren’t filtering things out efficiently. Doctors point out that taking strontium supplements with calcium at the same time might block absorption of both. Good advice: split the doses and don’t skip the check-ins with a healthcare provider. The Food and Drug Administration doesn’t treat supplements the same as prescription drugs, so not all brands stick to strict testing. Looking for products with quality certifications, like NSF or USP, gives more confidence about what’s in the bottle.
Strong bones matter at any age, especially if you want to spend more years upright and spirited, instead of sidelined. Strontium citrate is one of several tools. The best bone health stories usually feature a mix of steady movement, plenty of leafy greens, and maybe a little sunshine. Some folks benefit from adding strontium citrate based on bone scans and family history. The strongest evidence so far comes from long-term trials focused on bone strength. It’s not a “magic bullet,” but for people looking to boost their skeleton’s staying power, it brings another option worth talking over with the doctor.
Questions pop up all the time about supplements, especially ones that promise stronger bones or relief from chronic aches. Strontium citrate lands in that group. Plenty of people with bone density troubles—like osteoporosis—spot it on store shelves or in online ads. It’s easy to get drawn in by the idea that something outside the usual calcium and vitamin D might help. The safety of strontium citrate deserves careful attention before anyone fills their pill organizer with it.
Strontium, the mineral inside strontium citrate, shows up naturally in food, water, and even our bones at trace levels. The version found in supplements resembles calcium in many ways, and that’s why some folks believe it could help bones stay strong. In Europe, doctors sometimes prescribe a related compound—strontium ranelate—for osteoporosis. The science there actually shows some improvement in bone mineral density.
The story changes a bit in the United States because the FDA hasn’t approved any strontium compound for osteoporosis. Here, folks buy strontium citrate over the counter, mostly without a doctor’s say-so. Lots of supplements float around without rigorous government oversight. I’ve learned from years covering nutrition news that this situation calls for an extra dose of skepticism. Less regulation means ingredients might not always match what the label promises and side effects sometimes go unreported for years.
It’s tough to ignore the warnings from big-name groups. The European Medicines Agency flagged strontium ranelate for a higher risk of blood clots and heart problems. Even if the citrate form is different, no long-term studies have tracked its safety in large groups. Not all minerals behave the same way, even when they come from the same family table on the chemistry chart. Strontium at high doses pushes calcium aside in bones. Lab results raise concerns about weaker bone structure if strontium crowds out calcium over time, especially if someone keeps popping those pills without checking with an expert.
The National Institutes of Health lists strontium as “likely safe” at the tiny doses you might get from food or water. Go beyond that, and the evidence gets shaky fast. Regular use, especially in higher dosages, creates gaps in what researchers actually know. Anyone with kidney problems, blood clot history, or heart risk factors faces even more uncertainty.
In real-life practice, doctors, pharmacists, and dietitians spend much of their day talking patients away from risky supplements. Strontium citrate falls in that gray area where proven benefits stay narrow, but potential downsides loom large. If bone health is slipping, better solutions sit on the table: weight-bearing exercise, a steady routine with proven calcium and vitamin D, and medically-monitored therapies. Bringing questions to a primary care provider or a board-certified endocrinologist can make a world of difference in outcomes and safety.
What matters most comes down to informed choices. Sifting through the noise of online claims, anecdotal success stories, and slick marketing takes effort. People deserve clear facts and honest talk—especially when health and safety are on the line. Any supplement promising big results needs scrutiny, but especially those like strontium citrate, where the supporting research just doesn’t run deep enough to warrant careless use.
Strontium Citrate has earned attention from people interested in bone health, especially those worried about weak bones or loss of bone density. This supplement stands out because, unlike many calcium supplements, it seems to help build bone by slowing the cells that break down bone and encouraging those that help rebuild it. Yet, the way you take strontium can shape your results, for better or worse.
Look for products that list only strontium citrate as the main ingredient. Some brands mix in calcium, but that actually works against you. Calcium and strontium travel through the gut with the same transporter, which means your body might absorb less of both when taken together. Ideally, stick with a single-ingredient, third-party tested brand—NSF and USP seals matter because they show someone independent checked the purity and dosage.
Strontium citrate pulls ahead when you take it at the right time. Most nutritionists recommend taking it on an empty stomach, which means about two hours after eating or an hour before your next meal. My own experience and studies agree: mixing strontium with food—or calcium—means your gut grabs less of it. Focus on this timing if you want your supplement to actually do its job.
Most capsules come in 340-680 mg. Many research studies used up to 680 mg daily (measured as elemental strontium, not total weight of the compound). Talk to a knowledgeable doctor before starting, especially since too much can throw off the balance of other minerals in your body. For instance, excess strontium might mess with calcium and vitamin D, which could undercut your health in ways you don’t want.
Strontium should fit into a larger diet that covers calcium and vitamin D needs, but remember to space out those supplements. Take strontium at night, perhaps right before bed, and leave your daily calcium and vitamin D for morning or afternoon. This routine helped me stick with my supplements and avoid interference. Foods rich in vitamin K2 and magnesium also help—think leafy greens, nuts, seeds, and some cheeses.
Folks with chronic kidney disease or a history of blood clots should avoid strontium supplements. In my years of talking to both patients and peers, stories about kidney strain or clots traced back to improper supplement use show up more than you’d expect. Always mention strontium to your doctor, especially if you take other medications or deal with frail bones.
A lot of the buzz around strontium citrate comes from European studies—mainly using a prescription form not sold in the U.S. The over-the-counter citrate version shows some promise, but it’s not a cure-all. If you notice any side effects, odd heart feelings, or develop leg pain, stop and call a health professional. Instead of hunting for miracle solutions, combine strontium with consistent exercise, a balanced diet, and routine check-ins with your doctor. Trust your body’s cues as you make decisions.
Strontium citrate holds promise for people struggling with bone strength. Follow dosing guidelines. Keep it away from your calcium. Seek medical advice if you have any doubts. By paying attention to details, you give yourself the best shot at keeping your bones healthy, while avoiding unwanted problems down the road.
Strontium citrate has often been promoted as a supplement that keeps bones healthy, especially for folks trying to guard against osteoporosis. The idea makes sense at first glance—calcium gets most of the spotlight, but strontium belongs to the same family of elements, so maybe it can help too. There’s some limited evidence that strontium ranelate, a prescription drug (not a supplement), helps bone density. What’s being sold over the counter in the US is strontium citrate, which hasn’t been studied with the same scrutiny or in strict medical trials.
From years of paging through medical journals, a few things stick out. The biggest is this: most of the research looks at strontium ranelate, not the citrate version in supplements. Companies often lump the two together in their advertising, but our bodies may respond differently to each form. Researchers studying strontium ranelate have pointed out side effects. Some of these are mild and expected—nausea, diarrhea, headache, a metallic taste after taking the pill. Those might be annoying but not dangerous for most people.
One thing can’t be ignored. European health authorities have flagged rare but serious risks linked to strontium ranelate, like higher chances of blood clots and heart issues. While nobody has shown the same pattern for strontium citrate (mostly because it hasn’t been studied enough), the theoretical risk seems real. In fact, in the European Union, strontium ranelate now comes with restrictions because of these findings. Strontium can swap places with calcium in bones and blood vessels, and nobody really knows the long-term consequences of this in supplement form.
Here’s a detail worth remembering: Strontium is heavier than calcium. It makes bones look denser on scans, but not necessarily stronger. This can fool both patients and doctors, who might think bone density has improved, while the fracture risk stays the same. This trick of the eye is misleading, and it comes up in several studies. Normal healthy bone depends on more than just what shows up in a bone density scan.
Doctors advise people with kidney problems, a history of blood clots, heart disease, or who are pregnant or breastfeeding to skip strontium altogether. Children should stay away from it, too. The supplement interacts with some medications, including some heart and seizure drugs. Without good studies showing safety, people in these groups take an unnecessary risk by self-medicating.
Getting enough calcium and vitamin D from food or science-backed supplements, regular weight-bearing exercise, less smoking and alcohol—that’s the practical recipe for stronger bones. If someone’s truly worried about fracture risk or weak bones, a qualified doctor should assess whether a prescription medication fits. No over-the-counter bone pill does what lifestyle and medical oversight can offer.
Before adding something like strontium citrate to the routine, it’s smart to speak with a healthcare provider who follows clinical evidence. Supplements aren’t as well regulated as prescription drugs. Side effects don’t always show up right away, and most bottles come with little real guidance. Rooting decisions in fact, not hype, protects both bones and peace of mind.
Strontium citrate shows up in the nutrition aisles as a bone health booster. A lot of people think of it as a natural way to keep bones strong, especially for aging folks or anyone worried about osteoporosis. Sometimes, the urge to fix bone loss pushes us to grab every supplement we hear about, but strontium citrate does not work in a bubble. Combining it with other stuff in your cabinet can make some real differences, both good and bad.
Our bones carry both calcium and trace bits of strontium. Some experts highlight that strontium works like a double agent — it slows the breakdown of old bone and helps make new bone at the same time. But it is not interchangeable with calcium. The gut can only absorb so much mineral at once. If you swallow strontium with a big dose of calcium, they end up fighting for a ride into your system. Less gets absorbed of each, and your bones might not get the help you expect. I remember talking with my own doctor about supplements. She pointed out that people sometimes soak up less benefit because they do not pay attention to what competes during digestion. Strontium works best away from calcium — experts often say take them several hours apart.
Plenty of us take regular medications. Think about drugs to lower blood pressure, heartburn meds, even antibiotics. Strontium can mess with some of those. Proton pump inhibitors, common for reflux, can change how much strontium you soak up. Blood thinners pose other problems: If you have to avoid certain minerals to keep your blood right, adding strontium without talking to your doctor invites trouble. Strontium can also throw off lab tests, making it look like calcium levels are high when it’s actually just the strontium showing up on the radar. This matters if your doctor is using those numbers to fine-tune your treatments or spot new problems.
Supplements do not go through as much safety testing as prescription drugs. It seems harmless to toss curcumin, magnesium, vitamin K2 or D3 in the mix with strontium, but the body only has so much absorption power. Both vitamin D and magnesium play roles in how you bank minerals in bones. Overloading the system at the same time can blunt absorption — meaning you waste money, and sometimes you overload your kidneys. I have friends who take “bone health packs” with strontium, calcium, vitamin D, and magnesium all together, without realizing much of it passes right through. Facts from studies show that staggered dosing (spreading pills through the day) solves a lot of this problem. Simple, but most people forget in their morning rush.
Doctors and pharmacists train to spot weird interactions that most folks won’t know about. More than once, I have heard stories where someone mixed supplements and ended up with new health issues instead of stronger bones. Communicating with healthcare providers brings a real pay-off — they weigh your other meds, look at your lab numbers, and pick a safe plan.
Anyone trying strontium citrate for bones should look at the rest of their schedule: split calcium and strontium by several hours, check for conflicting drugs with a pro, and do not mix a huge stack of supplements on an empty stomach. Get labs checked regularly, track any side effects, and never swap strontium for prescribed medication without a clear conversation. Solid habits — eating balanced meals, staying active, keeping up with doctors — will always give you better odds than relying on any one pill, no matter how promising the label sounds.
| Names | |
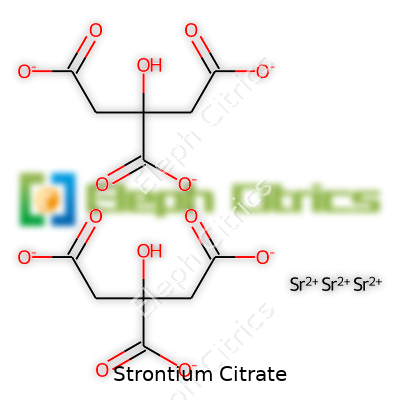
| Preferred IUPAC name | strontium 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Strontium 2-hydroxypropane-1,2,3-tricarboxylate Strontium(2+) citrate Strontium(II) citrate |
| Pronunciation | /ˈstrɒn.ʃi.əm ˈsɪ.treɪt/ |
| Identifiers | |
| CAS Number | 104897-77-0 |
| Beilstein Reference | 3583217 |
| ChEBI | CHEBI:86456 |
| ChEMBL | CHEMBL4298841 |
| ChemSpider | 21378463 |
| DrugBank | DB09315 |
| ECHA InfoCard | 100.029.194 |
| EC Number | 5706-54-3 |
| Gmelin Reference | 146116 |
| KEGG | C18794 |
| MeSH | D013320 |
| PubChem CID | 159729 |
| RTECS number | WL3676000 |
| UNII | 6K6976SE71 |
| UN number | UN2910 |
| CompTox Dashboard (EPA) | DTXSID6040637 |
| Properties | |
| Chemical formula | Sr₃(C₆H₅O₇)₂ |
| Molar mass | 627.7 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 0.9 g/cm³ |
| Solubility in water | Slightly soluble |
| log P | -1.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 7.7 |
| Basicity (pKb) | 9.3 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Dipole moment | 7.2 D |
| Pharmacology | |
| ATC code | A12GX05 |
| Hazards | |
| Main hazards | May cause respiratory irritation. May cause eye irritation. May cause skin irritation. |
| GHS labelling | GHS labelling: Signal Word: Warning; Hazard Statements: H319: Causes serious eye irritation; Pictograms: GHS07 (Exclamation mark); Precautionary Statements: P264, P280, P305+P351+P338, P337+P313. |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | May cause respiratory irritation. |
| Precautionary statements | Keep container tightly closed. Store in a cool, dry place. Wash hands thoroughly after handling. Avoid breathing dust. Use only with adequate ventilation. Wear protective gloves/protective clothing/eye protection/face protection. |
| NFPA 704 (fire diamond) | 1-0-0 |
| Explosive limits | Non-explosive |
| Lethal dose or concentration | LD50 Oral - rat - 3,120 mg/kg |
| LD50 (median dose) | 2296 mg/kg (rat, oral) |
| PEL (Permissible) | 10 mg/m³ |
| REL (Recommended) | 1000 mg per day |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Calcium citrate Magnesium citrate Strontium carbonate Strontium chloride |