Thiolactic acid, known chemically as 2-mercaptopropanoic acid, started attracting attention in the early 20th century. Researchers looking for ways to blend organic acids and sulfur noticed that thiol-containing acids behave differently than typical carboxylic acids. The presence of the thiol group infuses the molecule with unique reactivity. Thanks to pioneering studies by organic chemists trying to unravel sulfur metabolism, thiolactic acid stepped out from the background of academic papers. These foundational works identified routes of synthesis from lactic acid and mercaptans, setting the tone for decades of analytical, synthetic, and industrial attention. Many in the scientific community consider it an essential building block for creating specialized chemical intermediates and diagnostic reagents.
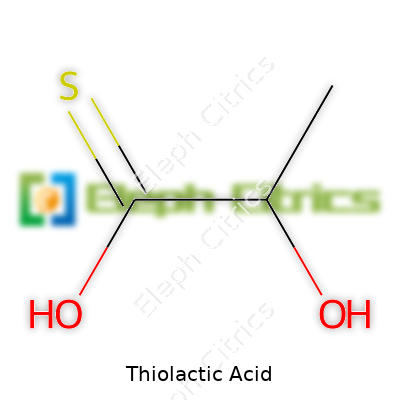
Thiolactic acid carries the structural formula HSCH2CH(CO2H). This solid at room temperature emits a pungent odor, which long ago made those handling it recognize the need for strong ventilation. Chemists respect its role as a multifunctional building block—its carboxyl and thiol groups let it link up with metals, organic molecules, and proteins in a way few small molecules can. Its industry-grade forms differ in purity, solution concentration, and stabilization method with product names reflecting the manufacturer, like Mercaptolactic Acid, 2-Mercaptopropionic Acid, or simply MLA.
Thiolactic acid usually appears as a pale yellow oil or low-melting solid, strongly smelling of mercaptans, with a molecular weight of 106.15 g/mol. It dissolves well in water and ethanol thanks to polar functional groups. Thiolactic acid stands out in its class for a boiling point near 100°C at low pressure, though it decomposes before reaching much higher temperatures, reflecting the reactivity of the thiol group. It has a pKa around 3.6 (carboxyl) and 10.6 (thiol), which tells you how it ionizes in water—critical information for anyone planning to blend or separate it. It reacts readily with oxidizing agents, forming disulfides, and forms chelates with transition metals, a property organic and inorganic chemists both have worked to harness.
A commercial bottle of thiolactic acid usually carries purity levels from 95% to 99%. Typical specifications spell out moisture content, residue on ignition, color index (APHA), and a maximum permissible content of heavy metals, aligning with both industrial needs and regulatory rules. Labeling follows both chemical safety standards and transportation codes. Globally harmonized system (GHS) labels flag harmful inhalation risks and skin sensitivity, displaying hazard statements, precautionary guidance, and UN numbers for cross-border shipment. Product datasheets from leading manufacturers reflect all this detail, plus shelf-life recommendations based on storage temperature and ambient humidity.
The industrial synthesis of thiolactic acid starts from lactic acid or its esters, reacting with hydrogen sulfide or sodium hydrogen sulfide. Aqueous or organic solvents can drive the reaction, and strong acid catalysis pushes the equilibrium toward the desired product. If benzyl mercaptan is available, it teases out the thiol group cleanly, though most plants stick with directly available hydrogen sulfide. After synthesis, the final mixture goes through distillation under reduced pressure, stripping off impurities and unwanted isomers, to yield the clear, pungent acid sought by formulators. Some labs have improved the process with more selective catalysts, tying into green chemistry trends to reduce waste and hazardous byproducts.
Thiolactic acid’s structure gives it a split personality—both sulfur and carboxylic acid reactivity. It participates in acylation, esterification, and amidation. With mild oxidizing agents, it dimerizes into its disulfide, bis(2-carboxyethyl) disulfide, which itself garners attention as a crosslinker. Alkylation of the sulfur atom opens up routes to specialty ligands and organometallic complexes. In analytical chemistry, it binds heavy metals such as cadmium and lead, forming stable chelates, letting labs measure trace contaminants in water or biological tissues. Protein scientists look to this molecule when they need to modify cysteine residues; its small size helps nudge thiol-disulfide exchanges without disruptive steric effects.
Even seasoned chemists can get tripped up by thiolactic acid’s alternate names. You’ll run into 2-mercaptopropionic acid or α-mercaptopropionic acid in old monographs. Suppliers might market it under abbreviations like MLA or as 2-Mercaptolactic Acid, and less frequently, Lactic Acid, 2-thio-. Researchers in biotechnology have shorthand like MPA for quick notes. This mess of synonyms reflects how different industries and generations have brought this molecule into their workflows, and can complicate inventory management if labeling isn’t crystal clear.
Anyone working with thiolactic acid must account for its volatility and toxicity. Inhalation can trigger headaches or nausea, and prolonged contact irritates the skin or even burns, echoing the risks familiar from other low-molecular-weight mercaptans. Ventilation remains priority number one in scale-up labs, not just fume hoods, but whole-building air changers. Glove selection matters, since the molecule can penetrate some plastics, so nitrile or neoprene wins out in practice. Storage requires airtight, dark glass bottles since both light and oxygen degrade it. As a hazardous substance, disposal routes involve oxidation to the inert disulfide or neutralization with strong alkali. Training covers not only emergency eye-wash but also real spill drills, raising the real-world stakes of carelessness.
Thiolactic acid’s real value emerges in practical chemistry and industrial processes. Chief among these, it acts as a chelating agent for trace metal analysis, key for environmental testing, where a few parts-per-billion of lead or cadmium make the difference between safe water and a health hazard. The pharmaceutical field respects its utility in peptide synthesis, especially as a protecting group for cysteine, keeping thiol or disulfide chemistry under tight control. Polymer scientists tap thiolactic acid for making specialized monomers granting reactivity to coatings, adhesives and ion-exchange resins. Certain cosmetic formulations rely on the same sulfur chemistry, giving perm solutions their curl-inducing power. Its interactions with biological systems, particularly enzyme and thiol-disulfide pathways, spur ongoing research highlighting why even a simple compound like thiolactic acid deserves careful study and control.
Current research circles pay close attention to thiolactic acid’s biological effects and new synthetic modifications. One wave of work delves into greener synthesis, avoiding hazardous waste streams and expensive reagents, trying out catalytic oxidation or enzymatic approaches. Analytical chemists push for even more selective chelating techniques using thiolactic acid derivatives. In drug development, formulation experts experiment with its interactions on protein surfaces, including enzyme inhibition or site-specific conjugation of drugs—tapping the unique reactivity of thiol and acid groups. Materials science teams tinker with thiolactic-crosslinked polymers, chasing after toughness, chemical resistance, and new sensor architectures. Even after a century of knowledge, practical researchers keep finding ways to bend its chemistry to problems in clean energy, diagnostics, and health.
Studies of thiolactic acid toxicity often focus on inhalation risks and dermal exposure. Animal models show acute effects similar to other low-molecular-weight thiols—transient central nervous system depression, mucous membrane irritation, and occasional lesions with high doses. Long-term exposure studies underscore respiratory irritation and sensitization, reminding us why it belongs in the ventilated, access-limited corners of the plant or lab. Regulatory agencies set workplace exposure limits based on these findings, though less data exists on chronic low-level contact than on short, high-dose bursts. Metabolic studies trace breakdown to lactic acid and sulfate, standard endpoints for many sulfur-containing organics.
The outlook for thiolactic acid stretches far beyond commodity chemicals or basic laboratory use. As industry prioritizes sustainability and specificity, thiolactic acid enjoys renewed attention for custom syntheses and as a base for specialty chelating agents. Its reactivity profile makes it ripe for advanced drug delivery systems or responsive coatings that change with the environment. Environmental chemists look to it as a rapid, cost-effective solution for heavy metal capture, both for remediation and real-time sensing. As biochemistry keeps revealing new protein modification pathways, the molecule finds broader use in proteomics and diagnostics. Far from a chemical footnote, thiolactic acid seems poised for expanded roles as industries demand more selectivity, safety, and sophistication in every stage from molecule to market.
Thiolactic acid doesn’t usually pop up in casual conversation, but anyone working around hair products or industrial chemicals has probably brushed up against it. Its unique chemical structure—combining a thiol and a carboxylic acid—gives it properties that make a real difference in a handful of products.
If you’ve ever sat through a perm, thiolactic acid may have helped transform your look. For decades, salons relied on ammonium thioglycolate, but thiolactic acid stepped in offering an alternative for people with sensitive hair or scalp. It drives the “breaking and reforming” of disulfide bonds in hair, which essentially lets a stylist reshape hair’s texture. Personal experience as a customer showed me the difference—my hair broke less, and the smell was far less aggressive. Professional stylists point out that customer comfort during perms improved once this milder option landed on the market.
Beyond beauty salons, thiolactic acid works behind the curtain in corrosion inhibitors and metal cleaning. It helps strip oxides from surfaces, revealing clean metal underneath. Industries banking on sensitive electronics or high-precision parts use it to keep things running smoothly. It’s not only about appearance—residues on metal surfaces can hurt performance. Chemical companies fine-tune the concentration to keep workers safe and results predictable.
Like lots of chemicals, case studies and research papers raise concerns about safe handling. Even with fewer users outside industry and salons, anyone who works with thiolactic acid should stay on guard. Overexposure to thiol-group containing acids can irritate skin or eyes. In the beauty world, salons train staff to follow strict protocols to keep customers and themselves out of harm’s way. In factories, ventilation and safety gear keep exposure in check. The materials safety data sheets underline these precautions for a reason.
Runoff from factories always tests a company’s commitment to the community. Wastewater containing thiolactic acid can damage aquatic life if it seeps into water supplies. Regulatory frameworks in most countries force companies to treat wastewater before discharge. From my experience working alongside environmental consultants, regular water testing and careful waste segregation cut risks—both to ecosystems and to company reputations.
Thiolactic acid delivered solutions where others fell short, but researchers push for even safer, greener options. The next frontier comes from bio-based or more biodegradable chemicals, hoping to offer all the perks with fewer environmental or health headaches. Early test results look promising, especially as consumer demand and tighter regulations raise the bar for safety and sustainability.
In the long run, learning about chemicals like thiolactic acid matters to consumers and workers alike. It empowers choices—whether that means picking gentler hair treatments or ensuring local water stays clean. It takes transparency from manufacturers and vigilance among end users to keep everyone safer.
Thiolactic acid often shows up in products for waving or reshaping hair—think perms and relaxers. This ingredient breaks sulfur bonds in hair, letting products create new styles that last. Chemists like its small molecular size because it reaches into hair fibers and acts fast. But the big question stares everyone in the face: Does all this chemical action stay friendly to our skin and locks?
Some industry groups and regulatory bodies keep a strict eye on ingredients, including thiolactic acid. The European Commission’s Scientific Committee on Consumer Safety looked at its use in cosmetics. It gave the green light for certain concentrations: up to 8% in hair wave products, up to 2.5% in depilatories and shaving goods, as long as instructions limit exposure to 10 minutes. The FDA, always quick to clamp down after bad incidents, hasn’t banned it—but watches for complaints and pushes companies to report adverse reactions.
Studies haven’t linked thiolactic acid to cancer or birth defects. In tests, skin irritation sometimes pops up, especially if anyone ignores timing instructions or uses a heavier dose than suggested. Allergic reactions count as rare, but they do happen. The smell can also be strong, making some users wrinkle their noses but does not, in itself, signal toxicity.
You can’t ignore that thiolactic acid lives close to skin, scalp, and eyes when used in hair and shaving treatments. In my experience as a consumer and conversation partner to salon workers, carelessness plays a big role in negative experiences. People skipping gloves or letting a perm sit too long end up with tears and itching. More than once, I’ve seen friends try “at-home” kits and regret going off-label with timing.
Reckless use or poor training raises risk. The professional beauty industry keeps logs of reactions and adapts. Good salons patch-test products before big applications. At the same time, plenty of home users go by brand trust, not label reading. If a product says “fast-acting,” curiosity or impatience sometimes trumps caution.
Thinking about thiolactic acid, I remember my aunt—a hairdresser—always yapping about the right gloves and timers. She warned me, as a teen, not to rush into any new perm gel before checking the ingredients and the instructions. For her generation of stylists, skin rashes in the 1980s taught painful lessons. Now, with more information at our fingertips, responsibility lands with both brands and users. Companies should label their products with clear directions, patch-test warnings, and contact details for adverse reactions.
Salons need to train staff in simple but vital safety steps: patch tests before service, close timing, and eye protection. Social media influencers have the power to push safe practices—posting realistic tutorials that don’t skip allergy checks or gloss over risks. Education keeps ingredients like thiolactic acid from turning an upgrade into an emergency room visit.
Major manufacturers research less harsh formulations—lowering concentrations, buffering solutions, or pairing ingredients with skin-soothing agents. EWG’s Skin Deep database and similar consumer tools help shoppers weigh risks and choose products based on sensitivity or past reactions. Dermatologist consultations offer a smart route for people with histories of eczema or allergies.
Thiolactic acid stays useful in beauty science, but products only work their magic when everyone—from companies to stylists to home users—respects the line between transformation and harm. Real safety comes from honest labeling, proper education, and customers paying attention—not just marketing or overnight success stories.
The substance known as thiolactic acid has a straightforward chemical formula: C3H6O2S. This compound pops up often among professionals in chemistry, biology, and even the manufacturing world. The ‘thio-’ bit marks the important presence of sulfur in the molecule, which sets it apart from lactic acid. Here you get a blend of the familiar lactic acid structure, with an OH group swapped out for an SH group—pushing the formula into a new territory, both chemically and in its applications.
Details matter in chemistry. Small tweaks, like swapping an oxygen atom for a sulfur atom, create very different substances. Lactic acid gets plenty of attention for its role in sour milk, muscle fatigue, and food preservation, but introduce sulfur, and the properties shift. Thiolactic acid brings a whiff of that rotten-egg smell you expect from sulfur compounds, but more importantly, it has its own set of uses in organic synthesis and industrial applications.
A lot of what we touch, eat, and use in daily life goes through fields where the right chemical formula makes all the difference. Take manufacturing processes. Workers in labs do not just pick any compound off the shelf. They verify the molecular structure fits the need. Thiolactic acid’s formula offers a precise combination for building blocks in pharmaceuticals, and sometimes it steps in for more specialized tasks, like chelation or fine-tuning pH in reaction vessels.
Anyone who works with chemicals quickly recognizes that even small differences in a molecule can change safety requirements. A memory comes to mind from a research internship, where handling lactic acid just meant basic gloves and goggles. Once sulfur entered the picture with thiolactic acid, it called for extra ventilation. The smell itself was a warning sign, and many of us learned the hard way not to underestimate chemical formulas. One letter, one atom—big change in how you approach storage and disposal.
Beyond the lab, safety data sheets and hazard training lean on exact chemical formulas to determine protocols. People’s health can turn on that information. Accidents in small labs and big factories remind us that confusion about a formula, or sloppy labeling, can quickly lead to exposure or contamination.
Teaching and learning in chemistry doesn’t stop once someone knows a formula like C3H6O2S. That knowledge has to be accessible. Printing the right data on containers, updating digital references, and making sure the information matches the real-world materials in use—these things keep people safe and help processes run smoothly.
It helps to see that reputable databases such as PubChem, Sigma-Aldrich, or peer-reviewed chemical literature list this formula, showing credibility behind the facts. People rely on trustworthy sources for accurate representations, especially students, new researchers, and workers just starting in chemical environments. Wrong information can ripple into costly mistakes.
Chemistry education would benefit from highlighting the importance of the exact chemical reality behind common names. Industry should push harder on training that ties formulas directly to on-the-ground handling. Software tracking chemical inventories could include formula checks, reducing human error at the point of storage and disposal.
Every detail in a formula adds up to safer workplaces, smarter research, and better products. Knowing the formula for thiolactic acid, understanding what it means, and keeping information current—these are not just habits for professionals, but a necessity for anyone working in science and manufacturing.
Everyday life brings us into contact with all sorts of chemicals, but most folks never stop to think about the bottles stacked in storage rooms and labs. Thiolactic acid isn’t as familiar as vinegar or bleach, but don’t let the name fool you. Used in cosmetics, hair perms, and chemical research, it carries a punch that deserves respect.
Heat speeds up reactions. Put thiolactic acid in a warm spot, and you’re asking for trouble. The compound can break down or release pungent fumes—trust me, nobody wants to breathe those in. I remember once working in a makeshift college lab during an unexpected summer heatwave. The bottles of chemicals felt warm to the touch, and the air stung my nose. Our advisor marched in and dragged a couple of containers to the basement. He knew those extra degrees could turn a manageable liquid into a risky mess.
Experts in chemical safety recommend a cool, dry storage space. Temperatures should stay steady, ideally somewhere between refrigerator-cool and typical room temperature. Fluctuations can stress the bottle. A simple wall thermometer keeps this in check, and you don’t need fancy gear—a basic fridge often works for small amounts, as long as food and drink stay far away.
Plastic won’t cut it. Thiolactic acid can eat through weak plastics over time. Use high-quality glass, preferably borosilicate, or chemical-resistant polyethylene. Tightly fitting screw caps stop vapors from sneaking out, keeping both the acid and users safer. I’ve seen labs try to stretch a dollar with questionable containers. A single leak left a yellowish stain on a shelf, and nobody wanted to clean it up. Over time, poor containment leads to bigger risks than just bad smells.
One of the biggest mistakes in storage comes from mixing things that react badly together. Thiolactic acid doesn’t play nice with strong oxidizers or bases. Two bottles side-by-side in a cramped cabinet, and even a small spill turns dangerous fast. Good storage practices mean placing incompatible chemicals on different shelves or cabinets.
Labeling comes next. A permanent label should list the chemical name, hazard symbols, and the date received. Dates matter because thiolactic acid ages. Over time, it can form buildup or lose effectiveness. A written log sheet by the cabinet tracks who grabs what and when.
No one likes to think about accidents, but preparation beats cleanup. Rooms for storage need steady, gentle ventilation—not a draft, but a way for vapors to escape. If you’ve ever worked in a building designed before safety rules mattered, you’ll remember the sharp odor when air sits still. A simple exhaust fan pointed toward the outdoors improves air quality by a mile.
Spill kits, eye wash stations, and gloves belong nearby. Regular training for anyone who handles thiolactic acid pays off in safety and confidence. Once, a friend’s quick use of an eyewash saved some serious tears during a splash incident. Good habits stop small mistakes from turning into emergencies.
Thiolactic acid never asks for attention. Still, safe storage deserves care—a cool spot, the right container, and proper training go a long way. Balancing safety, cost, and practical know-how helps keep both people and products in top shape.
Ask anyone who’s spent time in a formulation lab, and some will say thiolactic acid stirs up more questions than answers—mostly about compatibility. You start thinking about pH crashes, weird odors, and sudden precipitates just from blending the wrong stuff. Once, a colleague rushed in with a bottle of cloudy lotion—threw thiolactic acid in with some basic amines. Lesson learned: not everything can share the same bottle.
Getting compatibility right means less waste, safer products, and smoother production. In haircare and personal care, thiolactic acid tackles curl reduction, callus softening, and plenty more. Its chemical structure, with both thiol and carboxylic groups, makes it reactive—useful in breaking disulfide bonds in keratin, not so helpful if you want a long-lasting formula without surprises.
I’ve watched a batch go south after mixing thiolactic acid with a strong base like sodium hydroxide. The pH zipped up, and within minutes, the smell took over and the mixture separated. Strong alkalis neutralize the acid, break apart its active power, and leave the product weak or useless. On the other hand, oxidizers like hydrogen peroxide react fast with the thiol group, and suddenly you’re left with instability or unwanted byproducts. Hair relaxers use this chemistry, but it demands precision—otherwise, you end up with hot, unpredictable mixtures.
Formulators have luck pairing thiolactic acid with emollients, emulsifiers, mild surfactants, and buffered systems—especially at controlled pH close to acidic or slightly neutral. In my experience, using glycerin-based solvents soothes the raw smell. Thickeners such as carbomers remain stable if you balance the pH, and chelators like EDTA help bind stray metal ions, keeping the acid from reacting and changing color or texture.
Preservatives need careful selection. Parabens and phenoxyethanol keep up without sparking side reactions, while some organic acids lose strength or form unexpected odors. From trial-and-error, storing finished blends in airtight bottles works well, as thiols oxidize fast when exposed to air—leading to everything from loss of function to strong rotten smells that nobody wants near their face or hair.
Every time you develop something with thiolactic acid, test the mix under real-world stress. Move it between temperature extremes, let it sit for a week, check the phase stability and the odor. I’ve found that small changes in order of addition make or break a batch. Add thiolactic acid last and stir well; dump it in too early, and you risk an uneven reaction that won’t fix no matter how long you blend.
For brands looking at clean labels, there’s a tightrope walk. Thio compounds can trigger allergies at high doses, so skin-friendly concentrations matter. Narrowing ingredient lists to exclude harsh oxidants and strong alkalis keeps formulas safe and shelf-stable. Using packaging that slows down oxidation—like airless pumps—protects product quality, and regular microbial and chemical stability tests reveal weak links before launch.
Thiolactic acid opens doors for creative formulations, but it’s not a drop-and-go ingredient. Respect its quirks, favor gentle partners, and double down on testing for batches that deliver results and stay fresh on store shelves.

| Names | |
| Preferred IUPAC name | 2-sulfanylpropanoic acid |
| Other names |
2-Mercaptopropionic acid α-Mercaptopropionic acid α-MPA |
| Pronunciation | /ˌθaɪ.oʊˈlæk.tɪk ˈæs.ɪd/ |
| Identifiers | |
| CAS Number | '102-81-8' |
| Beilstein Reference | 87973 |
| ChEBI | CHEBI:38533 |
| ChEMBL | CHEMBL16337 |
| ChemSpider | 9322 |
| DrugBank | DB09312 |
| ECHA InfoCard | 100.005.091 |
| EC Number | EC 207-118-2 |
| Gmelin Reference | 6043 |
| KEGG | C02979 |
| MeSH | D013861 |
| PubChem CID | 69792 |
| RTECS number | OI8925000 |
| UNII | 6XM8G56G2H |
| UN number | UN2689 |
| CompTox Dashboard (EPA) | DTXSID7020379 |
| Properties | |
| Chemical formula | C3H6O2S |
| Molar mass | 106.14 g/mol |
| Appearance | Colorless to light yellow liquid |
| Odor | disagreeable |
| Density | 1.32 g/cm³ |
| Solubility in water | miscible |
| log P | -0.167 |
| Vapor pressure | 0.106 mmHg (25°C) |
| Acidity (pKa) | 3.60 |
| Basicity (pKb) | 11.55 |
| Magnetic susceptibility (χ) | -51.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.485 |
| Viscosity | 5.2 cP (20°C) |
| Dipole moment | 1.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 117.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -425.9 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -715.6 kJ/mol |
| Pharmacology | |
| ATC code | D10AX01 |
| Hazards | |
| GHS labelling | GHS05, GHS07 |
| Pictograms | GHS05 |
| Signal word | Danger |
| Hazard statements | H315, H318 |
| Precautionary statements | P210, P233, P280, P305+P351+P338, P309+P311, P403+P235 |
| NFPA 704 (fire diamond) | 2-2-0 |
| Flash point | 113°C |
| Autoignition temperature | 210 °C |
| Lethal dose or concentration | LD50 oral rat 2000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Rat oral 300 mg/kg |
| NIOSH | SW2975000 |
| PEL (Permissible) | 15 mg/m3 |
| REL (Recommended) | 10 mg/kg bw |
| Related compounds | |
| Related compounds |
Lactic acid Thioglycolic acid Mercaptoacetic acid 2-Hydroxyisobutyric acid |