Thiomalate traces its roots back to early investigations into sulfur-containing organic compounds during the 20th century. Back then, researchers chasing alternatives to existing arthritis medication stumbled across sodium thiomalate, which owes much of its background to broader gold compound research. French and British chemists ran trials in patients with rheumatoid arthritis—a time when options were scarce. Clinicians reported measurable improvements, giving thiomalate a reputation as a benchmark. Decades passed, and thiomalate held its position even as newer pharmaceuticals challenged its status. Through turbulent phases of research, false starts, and clinical observations, the therapeutic community steadily refined its understanding of thiomalate’s unique abilities, keeping it in the treatment conversation.
At its core, thiomalate describes a family of salts derived from mercaptosuccinic acid. Sodium thomalate stands out, reflecting the fusion between simple organic acid structure and a metal for stabilizing therapeutic ions. In practice, sodium thomalate found a spot in rheumatology, injected to limit inflammation in patients with chronic joint conditions. Over time, formulations adapted for differences in bioavailability or reduced dosing complications, but the primary attributes never shifted much.
This compound appears as a pale, crystalline powder. It dissolves well in water, which streamlines the preparation of injectable solutions. Chemists classify it under organosulfur compounds, carrying the signature odor and reactivity patterns. The presence of a carboxyl group and a sulfur atom arranges opportunities for both chelation and targeted reactivity in the body. Relative stability at room temperature allows for simple shipment and storage, but moisture protection remains necessary.
A pure sample lands typically at 99% minimum assay, and its aqueous solutions must pass strict microbial and particulate checks. Pharmaceutical standards require precise pH control, usually close to neutral, to prevent tissue irritation. Labels must spell out excipients, storage recommendations—ideally cool and dry conditions—and expiration dates. Batches need traceability, underscoring reliability from manufacturer to clinic. Regulations demand clear language about the gold ion content, reflecting clinical dosage standards established after years of trial, adverse event logging, and revised maximum limits.
Manufacturers synthesize thiomalate by reacting sodium hydroxide with thiomalic acid under controlled temperature and pH conditions. Purification follows, typically via crystallization from aqueous solution. Multiple washing steps remove byproducts, and drying under vacuum ensures a moisture-free end product. Quality assurance involves spectroscopic methods to confirm molecular structure, alongside chemical tests for residual reactants or potential contaminants. This method, refined over years, assures a steady supply for both research and clinical applications.
Thiomalate stands out as a ligand with rich coordination chemistry. Its sulfur atom forms stable complexes with various metals, not just gold. In reactions with oxidizing agents, thiomalate may lose its protective group, occasionally generating minor side-products during storage. Researchers sometimes chemically modify the acid backbone, aiming to tune its solubility or tissue distribution. Field trials with altered side chains seek to optimize the therapeutic window, reduce side-effects or create derivatives for targeted drug delivery.
Pharmaceutical catalogues list thiomalate under names like sodium thiomalate, sodium mercaptosuccinate, and under marketed trade names tied to injectable gold therapies. Each refers to the same active gold-sulfur core, although regional differences in formulation exist. Chemists and clinicians often swap terms, but proper labeling always reflects the internationally recognized monograph, so confusion rarely disrupts supply chains or patient care.
Safe handling relies on gloves, good ventilation, and eye protection, mainly due to the risk of allergic reactions reported by workers with long-term exposure. Clinical use protocols require slow intravenous administration, typically under observation. Caregivers monitor for kidney issues, swelling, or mouth ulcers—early signs of the rare but dangerous gold toxicity. Dosage charts, crafted over decades of patient monitoring, tightly control the total cumulative delivery to keep adverse events in check. Research labs keep spill kits and neutralizing agents nearby during synthesis or handling, as small leaks can lead to noxious fumes.
Use of thiomalate clusters most densely in rheumatology clinics, especially for patients with moderate-to-severe rheumatoid arthritis where standard drugs fail. Beyond medicine, research labs explore its chelating properties for analytical chemistry, where gold or silver extraction and purification call for its organic-sulfur backbone. A handful of companies even use thiomalate structures as starting points for other gold-based pharmaceuticals, treating conditions from autoimmune disorders to rare dermatological diseases.
Scientists dig deeper into the molecular dynamics of thiomalate, trying to understand precisely how the compound tames runaway inflammation in autoimmune disease. Results show its ability to suppress certain immune cell activities, sparking hope for broader application. Recent interest looks at nano-formulations, attaching thiomalate to metal nanoparticles for both targeted delivery and imaging. These developments could unlock new uses in precision medicine, imaging, or even cancer therapy, provided researchers account for toxicity risks and distribution in tissues.
Every new formulation or delivery method faces scrutiny from toxicologists. Classic clinical experience reveals rare kidney and bone marrow complications. Symptoms such as proteinuria, mouth ulcers, and rashes hit patients unpredictably and force discontinuation. Comprehensive studies run animal testing, then graduate to tightly monitored patient cohorts. Threshold dose calculations seek the balance between effect and risk. Modern research uses advanced imaging to track gold and thiomalate through organs, mapping where accumulations happen and how to minimize them. Ongoing toxicology pushes for predictive markers to warn doctors early, before irreversible harm begins.
Emerging trends suggest that thiomalate might pivot from its traditional arthritis role toward more tailored immunomodulation therapies. Advancements in chemistry open possibilities for derivatives with fewer side-effects or more precise organ targeting. As pharmaceutical industries chase smaller, smarter molecules, the underlying logic of gold-thiomalate—stable metal-organic frameworks—carries lessons for the next generation of biotherapeutics. Ongoing studies into safer forms and improved delivery could revive interest, especially for patients who have grown resistant or intolerant to mainstay drugs. Global demand remains, as unequal access to newer biologics in some regions drives clinical reliance on established compounds. Beyond medicine, environmental engineers and materials scientists continue to find roles for thiomalate’s chelating abilities in pollution control and metal recovery, suggesting a broader future footprint than traditional healthcare.
Many people have heard of gold-based therapies for arthritis, but not everyone knows thiomalate. Also called sodium aurothiomalate, this compound landed a spot in the medical field years ago. If you ask a rheumatologist who trained in the '80s, they’ll probably remember gold shots as a real option for managing severe rheumatoid arthritis. Thiomalate formed part of that gold standard back then—literally, because it contains gold.
Doctors used thiomalate to calm down inflammation in joints destroyed by rheumatoid arthritis. This illness doesn’t just cause aches; it can ruin hands, knees, everyday life. Before today’s wave of biologic drugs and disease-modifying choices, doctors injected thiomalate to slow joint damage and provide relief. My own family member took gold injections for a year before methotrexate became available. The improvement surprised everyone, but these shots also brought risks of skin rashes, mouth sores, and issues with blood counts, which forced us to monitor labs every month.
Gold-based drugs like thiomalate faded from most prescription pads with the rise of better, safer options. New drugs, better tolerated and just as effective, claimed their place in the treatment plan. Even so, some patients can’t take newer therapies. Older medications like thiomalate offer choices if nothing else works. Access to more treatment paths can be a game-changer for people with complicated medical histories or allergies to modern drugs.
Researchers haven’t abandoned thiomalate. Studies over the years have looked at its potential for other autoimmune diseases. Thiomalate sometimes comes up in rare situations where gold therapy remains the last resort. Most guidelines put emphasis elsewhere, but rare isn't the same as unnecessary. Once, a patient in my clinic couldn’t handle any oral therapy, and the only real option involved a gold injection protocol. It kept her active when everything else failed.
Any drug carrying real impact brings risk. Thiomalate can cause kidney trouble, changes in blood counts, mouth ulcers, and skin problems. That reality demands careful monitoring. Medical teams know how to spot side effects early, pausing or stopping treatment to avoid life-changing reactions. Regular reviews, blood tests, and a strong relationship between doctor and patient protect those taking gold-based therapies. In less-resourced areas, though, this level of supervision gets tricky, so access remains uneven.
To improve care for patients who still depend on thiomalate, updated guidelines help doctors decide when to give it, how to dose it safely, and which tests to run. Investment in telemedicine supports monitoring even at a distance. New research into modified versions might even cut down on side effects. As the push for more inclusive healthcare grows, reliable old medications—gold salt drugs included—offer hope when new ones fall short or aren’t available.
The story behind thiomalate isn't about living in the past. It’s about holding onto tools that still help certain people thrive today, all while pushing for safety, choice, and better treatments in the future.
Thiomalate, or gold sodium thiomalate, has a long legacy in the treatment of rheumatoid arthritis. For many, it brings hope when other medications do not control pain, swelling, and joint damage. The drug steps in as a disease-modifying antirheumatic, slowing arthritis’s creep into daily movement and basic comfort. Despite the relief this injection offers some, it comes with a series of side effects that require honesty between healthcare providers and patients.
After talking with patients over the years, I’ve seen how side effects from thiomalate can disrupt routines. Skin rashes often top the list. These rashes may look like simple irritation, but sometimes they flare up with itching, scaling, and redness that cover considerable patches of skin. Mouth ulcers aren’t rare, either; even a small sore on the inside of the cheek can make eating difficult and, over time, chip away at daily enthusiasm. Soreness, redness, or mild swelling at the injection site can linger for a few days.
Digestive troubles show up for some, too. Nausea may follow an injection, and though many power through, repeated bouts sap energy and patience. Diarrhea and mild stomach cramps carry their own frustrations, especially for older patients managing several medications.
Beyond these more familiar problems, gold therapy has an underside that must not get brushed under the rug. Gold compounds can build up in the body (gold toxicity), leading to kidney issues. Protein and blood appear in the urine, which no one wants to find midway through treatment. Without regular checks, patients can slip into a worse situation before a doctor catches on.
A drop in blood cell counts creeps in for some patients. Low white cell counts expose them to infections, while fewer platelets boost the risk of bleeding and bruising. These changes don’t always start loudly; they might go unnoticed until a blood test at a routine visit picks them up. Every so often, a patient experiences lung or liver issues. Persistent cough, yellowing of the eyes, or severe fatigue push back hard against the hope that treatment will make life easier, not harder.
Managing medication side effects means regular lab work and strong communication with a healthcare provider. Whole blood counts, kidney function tests, and urine checks can flag trouble before symptoms become overwhelming. Patients and doctors must stay alert together—complacency leads to missed warning signs.
In practice, honest conversations are the core solution. Physicians should sketch out potential side effects before the first shot—no sugarcoating, no rushing past uncomfortable facts. Patients armed with real knowledge can catch problems early, get help fast, and make a shift to other treatments if gold therapy becomes too harsh. With new biologics and oral therapies on the market, people living with rheumatoid arthritis deserve real choices, not surprises.
Thiomalate’s side effect profile means it rarely stands as a first choice today. Still, for some, its benefits may outweigh the headaches. That judgment depends on close monitoring and trust. Asking questions, reporting any odd changes, and sticking with routine labs form the backbone of safe use. For anyone considering gold treatment, a clear-eyed look at side effects protects both body and peace of mind.
Doctors and patients both care about how medicine gets from the bottle to the body. No one lines up for an injection unless they really need it, but sometimes that’s the best road to traveled health. Thiomalate—known mostly through its compound sodium aurothiomalate—has played a role in treating rheumatoid arthritis for decades. Wisdom from old-school rheumatologists sticks out here: the method matters just as much as the medicine itself.
Taking thiomalate by mouth won’t work. The stomach breaks down gold compounds long before they can do any good. Injections get thiomalate where it needs to go. Every shot lands deep into muscle, usually gluteal or deltoid, aiming for steady release and fewer local reactions. Doctors document every dose in charts, watching for gold deposits—one of those odd quirks with heavy metals—and adjusting if skin or eyes start to look yellow.
No one walks into gold therapy thinking side effects are a myth. The list runs long: mouth ulcers, rashes, even changes to blood counts. Routine urine tests check for protein—an early tip-off that kidneys aren’t handling things well. Regular blood work watches for falling white cells. Missing a sign puts patients at risk for bigger problems. Experience with long-term care has shown me the difference between careful monitoring and heading down a slippery slope.
Doctors start with small test doses, watching for allergies. In the first month, the amount inches up, once a week. If everything looks solid—no reactions, no fever, no drop in blood cells—maintenance doses come every couple of weeks. Some patients need more frequent shots during flare-ups. The goal is clear: keep joint swelling in check, give life back its usual rhythm, avoid piling up unwanted effects.
No one likes sitting in a clinic every other week, but skipping visits is risky. Self-injecting isn’t the answer; thiomalate’s narrow safety margin calls for trained hands and regular labs. In my experience, full honesty with patients on what to expect—bruising, joint pain from the shot itself, weird taste in the mouth—fosters better follow-through. Explaining why gold sticks around in the body, why blood and urine checks matter, why missing doses creates setbacks—all this makes patients see it’s not just medical red tape.
Many folks want pills or easier options. Methotrexate and biologics often take the lead these days, but not everyone can tolerate them. For some, gold injections remain an important option, especially where newer drugs aren’t available or don’t do the job. There’s room—always—for better treatments, safer delivery, less hassle. Until then, clinicians and patients need shared decision making, steady monitoring, and honest communication.
Keeping thiomalate therapy safe and useful calls for the whole team: doctors, nurses, patients, and their families. Teaching and regular check-ins give everyone the information and confidence needed to handle bumps in the road. By staying grounded in practical medicine and patient experience, health professionals can help make the process less daunting, more transparent, and, hopefully, just a little more manageable for those who count on it.
Anyone dealing with rheumatoid arthritis might find sodium aurothiomalate (a gold-containing drug, sometimes just called “thiomalate”) on their prescription list. This medicine helps dial down inflammation and pain when other treatments fall short. Since the drug has been used for several decades, doctors have seen both the ups and downs with it. Safety always comes first, especially during pregnancy or breastfeeding.
Pregnant and breastfeeding women face more hurdles than most when managing chronic illness. Many drugs that work for arthritis don’t mix well with pregnancy or nursing. Metals like gold can stick around in the body longer than other medicines, and that makes people worry about long-term effects on a baby. Scientists care about these chemicals passing through the placenta or into breastmilk and how much gets to the little one.
Researchers have only scratched the surface when it comes to studying thiomalate during pregnancy and breastfeeding. One reason is that cases with pregnant patients using gold therapy are rare, so there’s not much data. Still, the evidence that does exist points to risks. Older studies have linked gold drugs to miscarriages, birth defects, and blood cell problems in babies. Reports haven’t pinned down common, specific defects, but rare cases have raised questions. Safety agencies usually place thiomalate into the “not recommended” zone during pregnancy, barring desperate need.
During breastfeeding, gold can pass into milk. While only tiny amounts seem to reach the baby, kids have more sensitive bodies. A few case reports say gold showed up in infants’ blood, though no big health issues were proven. Still, the simple fact that gold builds up over weeks or months nudges most doctors away from using this drug with nursing moms.
Personal experience shows how hard it gets to balance arthritis and childbearing. Medication choices feel tighter, with fewer options and higher consequences. One patient once told me about the endless back-and-forth with her rheumatologist, searching for something safe before pregnancy. Joint pain hurt every morning, but she worried more about her child than her own discomfort. Many women end up switching to drugs like hydroxychloroquine or low-dose steroids, which have stronger safety records for pregnancy. Most experts prefer pausing thiomalate well before trying to get pregnant, just to avoid the risk entirely.
Open conversation makes a difference. Before planning a family, women should chat with both a rheumatologist and an OB-GYN to set up a plan. Sometimes, counseling with both specialists shows ways to control symptoms without relying on riskier medications. Blood tests and regular checkups can help cut out surprises, making it possible to manage flares in a safer way.
Patients deserve clear facts, not just warnings. Medical experts agree that older drugs like thiomalate make more sense as last resorts—not as regular options—when pregnancy or breastfeeding comes into play. Newer therapies, including biologics like certolizumab, have been shown safe in more studies and can provide strong relief. If pain takes over, it’s still better to tackle it with a medication that’s been tested more thoroughly in mothers and babies. Rheumatology keeps changing, and doctors today have better tools than ever before—no one needs to go through this journey alone.
Organizations like the American College of Rheumatology and the Centers for Disease Control list updated treatment guidelines on arthritis in pregnancy. Actual product labeling from gold-drug manufacturers also includes strong warnings about use in women trying to get pregnant, currently pregnant, or breastfeeding.
Thiomalate enters the scene mostly in medical settings, used for conditions like rheumatoid arthritis. This compound, which contains gold, packs powerful effects and risks. Growing up in a family with several chronic health conditions, I watched relatives navigate complicated medication routines—especially those drugs that could cause dramatic side effects. Medicines like thiomalate aren’t to be taken lightly. Dose missteps or poor monitoring can snowball into bigger health problems.
Before starting thiomalate, doctors always run checks on kidney and liver health. I’ve seen physicians insist on thorough blood work, especially for older relatives with pre-existing issues. The kidneys and liver process many medications, and thiomalate puts extra strain on these organs. Failing to spot lower kidney function ahead of time sometimes means trouble down the road, with risks ranging from severe skin reactions to organ damage. Never skip baseline tests or regular follow-ups; the consequences last longer than a single missed appointment.
Frequent monitoring, weeks and even months after starting treatment, catches problems before they hit hard. Blood counts, urinalysis, and kidney function stay on the list. I’ve heard stories from people whose mild symptoms turned serious before their next blood test; only swift action kept the situation from spiraling. Early warning signs—mouth ulcers, rash, odd bruising—deserve quick attention. Reporting them beats hoping they fade away.
Thiomalate can clash with other drugs. My grandmother dealt with this firsthand, managing a list of prescriptions after a lifetime of arthritis. Her pharmacist spotted a dangerous overlap that saved a hospital visit. Medications for hypertension, heart conditions, or other immune-suppressing drugs may amplify thiomalate’s side effects. Sticking with the same pharmacy and informing every healthcare provider helps keep combinations in check.
This medication usually goes by injection, never oral tablets. Only trained healthcare workers should give it, usually in a clinic. Any spills or skin contact with the liquid deserve careful cleanup. Gold compounds bring potential for allergic reactions, including severe rashes or breathing problems, so patients usually sit under observation after their shot. Personal experience says even people with high medication tolerance can react badly to new injectables. Better to wait for half an hour than rush back for emergency care.
Certain drugs like thiomalate dampen the immune system. Too many stories circulate in chronic disease communities about simple colds turning nasty. Extra hand washing, staying up to date with vaccinations, and avoiding unnecessary crowds during flu season become daily habits. A friend who forgot these lessons ended up with a hospital stay for an infection that wouldn’t clear. Solid prevention routines help avoid these setbacks.
Thiomalate is no minor player. It requires respect, communication, and close teamwork between patient and provider. As someone with family navigating tricky medications, I’ve seen that most problems start with missteps in daily routines—missed labs, shrugged-off symptoms, or skipped communications with the care team. Being proactive, speaking up, and embracing a partnership with healthcare professionals make treatment more manageable and far safer.
| Names | |
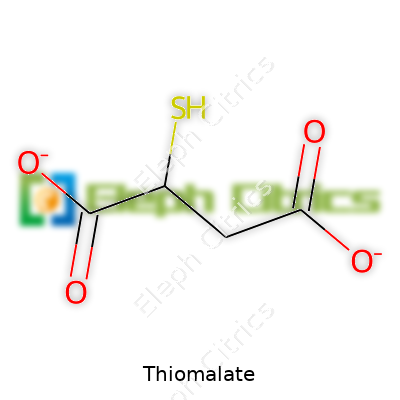
| Preferred IUPAC name | 2-sulfanylbutanedioic acid |
| Other names |
Thiomalic acid Thiomalic acid salt Mercaptosuccinic acid |
| Pronunciation | /θaɪˈɒməleɪt/ |
| Identifiers | |
| CAS Number | [40-93-9] |
| Beilstein Reference | 2954342 |
| ChEBI | CHEBI:135723 |
| ChEMBL | CHEMBL1426 |
| ChemSpider | 75795 |
| DrugBank | DB01394 |
| ECHA InfoCard | 100.018.158 |
| EC Number | 3.1.3.77 |
| Gmelin Reference | 145519 |
| KEGG | C02948 |
| MeSH | D013849 |
| PubChem CID | 52921686 |
| RTECS number | OV8575000 |
| UNII | X2T04D3C78 |
| UN number | 2811 |
| Properties | |
| Chemical formula | C4H4O4S2 |
| Molar mass | 226.18 g/mol |
| Appearance | White or almost white, crystalline powder |
| Odor | Unpleasant |
| Density | 1.98 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -2.1 |
| Acidity (pKa) | 3.3 |
| Basicity (pKb) | 12.48 |
| Magnetic susceptibility (χ) | -23.0e-6 cm³/mol |
| Refractive index (nD) | 1.629 |
| Viscosity | 660 cP |
| Dipole moment | 4.95 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 110 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | M01CB02 |
| Hazards | |
| Main hazards | Harmful if swallowed, inhaled, or absorbed through skin; may cause allergic reactions. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS05,GHS07 |
| Signal word | Danger |
| Hazard statements | H301 + H331: Toxic if swallowed or if inhaled. |
| Precautionary statements | P264, P270, P273, P301+P312, P330, P391, P501 |
| NFPA 704 (fire diamond) | 2-1-2-W |
| Lethal dose or concentration | LD50 (rat, oral): 960 mg/kg |
| LD50 (median dose) | LD50 = 165 mg/kg (rat, intraperitoneal) |
| NIOSH | WA2625000 |
| PEL (Permissible) | 0.01 mg[Au]/m³ |
| REL (Recommended) | 15 mg weekly |
| IDLH (Immediate danger) | IDLH: Not established |
| Related compounds | |
| Related compounds |
Penicillamine Mercaptopurine Gold sodium thiomalate |