Chemists started looking into titanium compounds back in the 19th century after titanium’s discovery in crude mineral samples. The world’s fascination with lightweight metals pushed many researchers to focus on titanium salts for industrial uses. Modern stories of titanium citrate began in the search for titanium chemicals that can dissolve in water and offer stable coordination for specialized processes. This compound didn’t catch the popular spotlight like titanium dioxide, but its unique blend of bioactivity and reactivity earned a home in several research and industrial labs. Many early records point to metallurgical research and analytical chemistry, where titanium citrate often surfaced in tests requiring a solubilized titanium source.
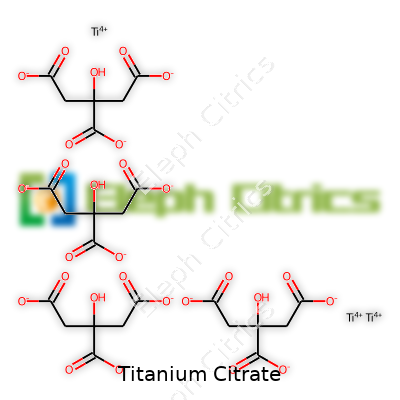
Titanium citrate forms a complex between trivalent or tetravalent titanium ions and citric acid. Depending on the synthesis and environmental conditions, you'll run into different structural tweaks. Usually, suppliers provide it as a slightly yellowish water-soluble powder or concentrate solution. For technical buyers and researchers, the product’s appeal lies in its capacity to serve as a titanium donor, either for analytical assays or for catalyzing certain chemical pathways. It’s not the type of compound you find in grocery store supplements or general industry blends. Instead, you see it on the shelf in research chemical stores and companies developing advanced materials.
You’ll recognize titanium citrate by its distinct yellow-green tinge and mild, sour hint—mostly due to the citric acid component left over after complex formation. Its solubility marks one of its biggest draws, making it useful in aqueous systems where most titanium oxides would fall out of solution. Chemically, the stability of the titanium-citrate bond changes with pH, temperature, and whether you’re dealing with Ti(III) or Ti(IV). That reactivity provides opportunities but also means process conditions demand careful attention. Its molar mass and density may depend on how hydrated the compound comes out of synthesis. In dry storage, it tolerates air for short periods but performs best under sealed conditions to avoid moisture shifts or oxidation, especially in Ti(III) states.
Marketed titanium citrate should list whether you’re dealing with a trivalent or tetravalent variant, since the chemical behaviors shift considerably between those two forms. Labels cover concentration, pH range for solution-keeping, and general storage guidelines. Purity isn’t just a technicality; even small deviations from minimum requirements could sway a reaction one way or another. Common numbers float above 98 percent for research grades, but trace analysis of heavy metals or organic residues can tip researchers off to possible interferences. Reliable suppliers also highlight moisture content, since water can affect both shelf life and certain applications. Shipping containers and bottle labels usually stress light shielding since extended exposure can lead to gradual decomposition.
Manufacturers don’t just mix powders and hope for magic. They start by dissolving titanium salts—usually titanium oxide or sulfate—in an acidic medium. Next, citric acid joins the system, and the whole mixture gets fine-tuned with pH adjustments. Temperature, mixing speed, and order of addition influence both the structure and final chemical profile of the titanium complex. Industrial preparation usually calls for inert gas protection, especially for Ti(III) variants, to avoid unwanted oxidation. Filtration and vacuum drying round out the steps, leaving behind either a powder or concentrated solution. For the highest purity, recrystallization or additional washing under controlled conditions eliminates unbound citric acid or metallic contaminants. It’s a dance requiring hands-on attention, not just following some paint-by-numbers routine.
In the lab, titanium citrate expands beyond just a stable salt. Scientists use it to create customized titanium-containing materials, acting as a building block for advanced ceramics or catalysts. It readily undergoes coordination exchange, giving chemists ways to swap out citric acid for other ligands, or to attach doping agents for improved catalysis or activity in sensor platforms. Reduction-oxidation shifts are tricky but manageable, with Ti(III) showing distinct electron transfer capabilities that come in handy for photochemical experimentation. Chemically, people see titanium citrate as a flexible precursor—bridging organic acids and inorganic frameworks for applications where titanium’s lightness and reactivity can shine. Hydrogen peroxide, mild bases, or organic solvents tap into specific chemical settings, leading to further downstream reactions and material modifications. In short, the compound rarely stays static, especially under creative hands.
Though technical chemistry circles stick to “titanium citrate,” you’ll find alternate names like “citrate of titanium,” “titanic citrate,” or “citrated titanium(III)” depending on source or supplier. Some catalogues assign the compound a code reflecting its oxidation state, such as “titanium(III) citrate solution.” Others prefer systematic names that stretch into wordy territory, like “tricitrate titanium complex,” but most people in the field just call it by shorthand. High-purity or customized grades pick up commercial labels tacked on by manufacturers, but clarity in documentation always trumps branding when it comes to sensitive research applications.
Working with titanium citrate isn’t like handling laundry detergent or store-bought vitamins. The compound’s acidity and potential for reactivity demand protective gear—gloves, safety goggles, lab coats. Inhalation or direct skin contact might lead to minor irritation in sensitive individuals, more so for concentrated solutions. Storage follows chemical standards typical for other metal-organic complexes: cool, dry places, away from direct sunlight and sources of extreme heat. To avoid contamination or degradation, bottles should stay tightly capped and checked regularly for changes in color or texture, which signal possible decomposition. Waste management needs attention—a lot of localities have restrictions for compounds containing transition metals. Responsive safety teams and emergency procedures make a difference should spills or accidental exposures occur. Reliable operations come from consistent adherence to protocols set by chemical safety boards and institutional rules.
Hardly any industrial-scale production lines use titanium citrate today, but it occupies a sweet spot in small-scale material research and production. In analytical chemistry, it finds use as a reducing or oxidizing agent for colorimetric assays or metal quantification. In advanced materials, the compound’s role shows up as a starting block for sol-gel processes, thin film deposition, and even nanoparticle synthesis. Sometimes, it helps tune the catalytic properties of titanium-based materials used for environmental cleanup or in energy tech like dye-sensitized solar cells. Biomedical research eyes titanium citrate both for its ability to deliver titanium ions under mild conditions, and for exploratory work involving metal-based pharmaceuticals. The compound even slips into specialty coatings and corrosion protection testing, mainly where standard titanium forms fall short in solubility or reactivity.
Academic labs push the boundaries with titanium citrate, diving into how it behaves under new coordination environments, or modifying reaction parameters to see new material outcomes. Ongoing studies probe the kinetic and thermodynamic stabilities of its metal-organic frameworks, looking for leads that could create better catalysts or biocompatible implants. Some chemists investigate its electron transfer pathways, hunting for innovative battery materials or photocatalysts that can outmaneuver conventional options. Pharmaceutical teams remain cautious, but a few run trials to see if titanium citrate complexes can serve as delivery vehicles for targeted therapy. Grants and industry-backed programs funnel investment into scaling up certain preparation techniques, hoping to bridge the gap between benchtop discoveries and industrial practicality. Wherever curiosity dwells, titanium citrate often lands on the reagent shelf, waiting for the next hypothesis or prototype.
One persistent question shadows every new metal-organic complex: safety for people and the planet. With titanium citrate, past studies reveal low acute toxicity under lab conditions, especially compared to heavier transition metals. Oral or dermal exposures at standard research concentrations produce minimal adverse effects, but ingestion of larger doses hasn’t been fully mapped out. Chronic exposure or use in consumer settings undergoes ongoing review—regulatory agencies still expect more thorough toxicological profiles before supporting non-laboratory uses. Environmental effects depend on wastewater treatment efficiency; titanium isn’t classified as a top-priority pollutant, but its organic complexes do alter local metal cycling if mishandled. Toxicologists echo a cautionary theme: respect the compound’s chemical power, inform yourself about evolving hazards, and never substitute anecdotal impressions for peer-reviewed results.
Looking ahead, titanium citrate sits at the crossroads of material innovation and sustainable chemistry. Trends in green synthesis and resource-efficient manufacturing push researchers to make the most out of soluble titanium complexes. Next-generation applications might include customizable catalysts, low-toxicity biomaterials, and high-performance coatings for specialized industries. Progress hinges on a better grasp of the compound’s reactivity under real-world conditions and tightly controlled manufacturing protocols. With continued advances in characterization technology and collaborative research, the narrative of titanium citrate extends well beyond its current niche, and may end up shaping how chemists and engineers solve tomorrow’s pressing problems. For now, its role remains dynamic—a material in motion, shaped by inquiry and imagination.
Titanium citrate shows up on ingredient lists in a few corners of life, but most folks will never come across it at home. This compound is a mix of a titanium salt and citric acid. Its main claim to fame sits in research or industrial labs, not kitchen cabinets or typical pharmacies. Still, it pops up in some medical and dental products, mainly down to its versatility and the things it can coax out of other metals or chemicals.
Researchers figured out that titanium citrate has a knack for keeping metal ions stable in solution, thanks to the way citric acid wraps around them. This trait lands it in a supporting role for oral hygiene and dental care. Some toothpastes and mouthwashes make use of titanium citrate as a mild abrasive and a stain-remover. The idea isn’t about whitening teeth with a chemical blast, but about gently polishing away debris without damaging enamel.
Beyond teeth, the story heads straight for the lab. Chemists and biochemists use titanium citrate for its reducing potential—it donates electrons. This makes it useful in experiments that measure antioxidant activity or that test how something responds to oxygen. For example, certain tests for cell health, enzyme reactions, and DNA research lean on chemicals like titanium citrate to create very controlled reactions. Without these types of compounds, results would show up muddy or outright misleading.
Energy and materials scientists also look at titanium citrate for the way it can make metal nanoparticles. By managing how metal ions clump and react, this substance helps create tiny structures for catalysts or battery parts. Such tiny particles don’t happen easily; the citrate acts almost like a traffic manager to keep things orderly.
Folks care about what ingredients go into health products, especially in their mouths. While titanium dioxide, a different compound, has seen widespread scrutiny for its potential health risks when inhaled long-term, titanium citrate operates differently and is used in much lower amounts. Most evidence suggests it sticks around for only short stretches and gets washed away easily. The risk drops even more when used on teeth rather than breathed in or swallowed.
There’s an argument to keep an eye on any compound that enters the consumer product space, especially new or unfamiliar ones. The skin and the inside of the mouth absorb chemicals faster than, say, the outer layers of your hands. Regulators and manufacturers have a responsibility to publish research, run more long-term safety trials, and be transparent about testing practices. Academic journals and independent labs often push for this, as public trust depends on it.
Anyone interested in the safety of dental products with titanium citrate can look up published toxicology studies or reach out to their dentist. The best option is always to choose products from reputable companies, especially those that publish full ingredient lists and fund proper research.
On the science side, ongoing monitoring and updates from food and drug safety agencies play a big part. Advocates and researchers should push for open data so that anyone—consumer or chemist—gets reliable answers. Responsible use isn’t just about what works, but about understanding the long-term impact for people and the environment. With good science and steady communication, titanium citrate can stay helpful where it belongs.
Titanium Citrate might sound scientific, but questions about its safety for human consumption aren’t just for people in white lab coats. From food additives to supplements, ingredients matter. I always check food labels, not out of paranoia, but because experience taught me that hidden chemicals can leave their mark on our health.
Titanium Citrate isn’t a household name, but it crops up in niche products. The compound includes titanium, a metal not usually seen in our diet, paired with citric acid, something every orange can boast about. Most people know about titanium dioxide, used as a white pigment in everything from toothpaste to candy. Titanium Citrate, though less common, raises many of the same questions.
Titanium itself resists corrosion, which makes it great for medical implants. Inside our bodies, though, titanium ions behave differently. Researchers have checked for potential toxicity and bioaccumulation, especially since titanium dioxide’s use in food caught flak in Europe. In 2022, the European Food Safety Authority flagged uncertainty about titanium dioxide’s ability to damage DNA. France already banned it as a food additive. While Titanium Citrate isn't exactly the same molecule, the presence of titanium in any compound still brings up legitimate safety concerns.
No one wants to play lab rat with their health. My personal policy is to steer clear of any additive if scientists can’t agree on its safety. There are animal studies examining the impact of titanium salts and related compounds. Some studies point to low absorption through the gut, with much of the substance just passing through. Other work shows titanium can build up in certain tissues over time, raising eyebrows in the toxicology world.
Long-term data in humans are thin. No clear links to cancer or acute diseases came out of general population studies, probably because not many people knowingly consume titanium compounds every day. Still, what sticks with me is how regulatory bodies often say there’s not enough high-quality evidence either way. This uncertainty means that possible risks from chronic, low-level exposure are hard to rule out.
Anyone shopping for supplements or processed food needs practical answers. Trust matters. I only buy brands that list every ingredient and back up safety claims with third-party testing. Transparency goes a long way, but clear, up-to-date research matters more. If titanium compounds show up on a label, I’d rather ask the company for recent safety test results than rely on a vague “generally recognized as safe” claim.
Manufacturers hold the power to push for safer, well-researched alternatives. Plant-based colorants or functional food additives draw less suspicion because their history of safe use speaks for itself. The industry can collaborate with scientists and check each ingredient for real-world health impact, not just whether it meets a regulatory definition.
Policy makers have a role, too. Regulators can fund long-term, independent studies to close gaps in our understanding. Consumers deserve the truth, but industry transparency follows when clear rules hold everyone to the same standard. While the world waits for final scientific consensus, caution and curiosity both belong at the kitchen table.
Most folks have never really thought about titanium citrate. The compound shows up in some supplements, research, and industrial applications, but rarely ends up in the news or conversations around the dinner table. Anyone who researches ingredients for health or safety reasons may stumble across this one and wonder what side effects it brings.
Supplements raise questions and titanium salts fall into a muddled area between tried-and-tested ingredients and unknown territory. Direct research on titanium citrate isn’t widely published, but lessons from related titanium compounds and case reports shed some light.
Gastrointestinal trouble probably ranks at the top of the list of reactions. I’ve read anecdotal reports of nausea, stomach cramping, and mild diarrhea in some individuals taking titanium-based supplements. These sorts of reactions show up fast, usually as the body tries to figure out what’s been ingested. Given that titanium itself has no known biological role, it’s not surprising if the digestive system objects.
Lab research offers clues about toxicity. The European Chemicals Agency notes that titanium compounds — including titanium dioxide — can cause inflammation if inhaled or ingested in large quantities. There’s no established nutritional need for titanium, so people who have allergies or sensitivities to metals might feel worse, not better, with it in their system.
Nobody should ignore the potential for heavy metals to build up in the body. Ongoing exposure to titanium citrate might increase the risk of chronic kidney issues, as the kidneys act as a filter for metallic compounds. Research in animals sometimes points at organ accumulation, which sparks concern. The risk grows for anyone with pre-existing kidney trouble or those who take multiple mineral supplements.
A few researchers have looked for links between titanium exposure and neurological symptoms. Rare animal studies suggest behavioral changes after large or long-term dosing, though it’s hard to say exactly how this translates to humans. No regulatory agency has approved titanium citrate for routine human consumption, and the FDA demands real safety data before it gives a green light.
I’ve met plenty of people who try new supplements before reading up on possible effects. Titanium citrate belongs on the list of additives that deserve caution, especially for anyone with allergies, kidney issues, or a record of stomach problems. Pregnant mothers, children, and those on multiple meds all have a stronger reason to ask questions first.
Side effects don’t affect everyone the same way. The dose, duration, and a person’s health shape the risk. If your gut, skin, or energy levels change after trying a new vitamin or mineral, it’s smart to stop and talk to a health professional. Labs can test for metal buildup, and doctors keep up with the latest findings from clinical case reports.
The safest move comes down to evidence. Until safety trials in humans show no risk, most providers recommend steering clear of titanium citrate for everyday use. Following trusted sources — like advice from the FDA, EMA, or leading clinicians — helps cut to the facts and keep supplements safe. If a company won’t list side effects or back up marketing with studies, it should raise suspicion.
Staying informed means checking sources, following science, and making choices that match your health goals and comfort zone. The world of supplements keeps growing, but nothing beats a well-informed conversation with someone who understands your medical history.
I get a lot of questions about minerals and supplements. Titanium Citrate, in particular, shows up on lists, sometimes with big promises but little detail. It isn’t something you’ll find stacked next to multivitamins at your local pharmacy, and it definitely isn’t a regular part of most people’s supplement routines. Before reaching for anything new, checking what research says and what safety data exists always makes sense.
No one wants to play guessing games with their health. Titanium isn’t an essential nutrient in the diet, and citrate is a common compound, often found hooked to minerals because it helps with solubility. The catch: there’s very little science exploring how much Titanium Citrate a person should take, or even if it offers real benefits compared to established minerals like zinc or magnesium. The lack of clear guidance comes from a lack of large, credible human trials. Going by anecdotal reports or internet forums can be risky—everybody’s biology differs, and so does their reaction to unknown substances.
Supplements should always get the same respect as prescription pills. Anything you put in your body has the potential to do harm, especially substances that don’t play a known role in human nutrition. With Titanium Citrate, health authorities like the FDA haven’t set a daily intake or dosage. That fact stands out. Most well-researched nutrients have established tolerable upper levels; titanium doesn’t fall in that camp. The European Food Safety Authority, the FDA, and other bodies have raised questions about titanium compounds (often as colorants in foods), not for supplementation.
Any instructions you do find online, like taking it in the morning or with food, are unproven and usually based on general supplement habits, not science. If a manufacturer supplies a label with dosage information, approach it with healthy skepticism. It’s smart to ask: “Who made these recommendations? Was a qualified researcher or registered dietitian involved?”
Too many people think “natural” products can’t do harm. The truth: lab-made compounds without clear benefits or safety testing can be a problem. Titanium compounds can accumulate in tissues and haven’t been shown to offer a health benefit in reputable human studies. Anyone with kidney, liver, or autoimmune issues should be especially cautious; unknown substances could stir up unexpected trouble.
I’ve seen cases where someone starts a new supplement, convinced it’ll make a difference, and winds up with odd symptoms traceable to a lack of safety data. If you’re thinking about Titanium Citrate, ask a pharmacist or physician to weigh in. They base their advice on what’s proven, not just what’s trending online.
If there’s a specific mineral need, ask for a blood test. Work with a healthcare professional to target nutrient gaps with products that have documented benefits and researched dosing. For general health, focus on time-tested choices: balanced meals, activity, and hydration. Uncommon supplements usually take a backseat to fundamentals.
Food and supplement labels can make even the most informed shopper scratch their head. Titanium citrate falls into this camp. For anyone following a vegan or vegetarian lifestyle, ingredient lists become a maze. You either trust the name, ask questions, or put the package back on the shelf. Titanium citrate often pops up in discussions about colorants and mineral sources, so its origins and processing matter a lot to anyone avoiding animal products.
Titanium, as a mineral, comes straight from the earth. Companies extract it from ore, not from plants or animals. Citrate ions in food and supplements usually get produced from citric acid, derived by fermenting sugar using microbes like Aspergillus niger. This process doesn't touch animal-derived materials, at least at the initial chemical level.
On paper, both compounds look vegan-friendly. The catch: the production process isn’t always crystal clear to the average consumer scanning a label, especially when supplies can vary between manufacturers or countries. Not every company lists their sourcing details. Sometimes, auxiliary agents or cleaning steps in factories use animal-derived substances. That part stays hidden from public eyes, unless the brand chooses to be transparent.
Experience tells me that just because a chemical compound starts out animal-free does not always mean the finished ingredient stays that way. Some supplements I have checked for friends over the years carried a vegan or vegetarian logo, while others dodged the question completely. Essay-length ingredient lists can create more confusion than clarity. I learned early on that titanium dioxide, a cousin of titanium citrate, rarely involves any animal product in its creation, but concerns shifted to the possibility of contamination or hidden binders. That suspicion translates here, too.
From time to time, reports surface about supplement capsules using gelatin or companies sharing production lines with animal-based products. Many supplement capsules now use cellulose, which sidesteps animal involvement, but not every brand makes that switch. Some certifications focus on the main active ingredient but skip the “other ingredients.” The titanium citrate inside the supplement may pass the vegan check, but if it's wrapped in gelatin, the whole product no longer does.
No government body forces companies to report the finer details of mineral sourcing, especially for compounds like titanium citrate. A group of certified vegan brands exists, but plenty of generic formulations sit on store shelves. Confidence often boils down to trust and thorough research. Some brands voluntarily get their products certified by groups like The Vegan Society or Vegetarian Society, which run supply chain checks. Labels featuring these logos typically mean every step of the process has been vetted.
The safest route involves looking for products marked specifically suitable for vegans or vegetarians, or contacting the brand directly for details. More companies now respond to these requests, understanding that shoppers want ethical clarity. Third-party testing and clear communication help build trust. Checking for industry certifications and asking tough questions can help avoid animal-derived surprises.
Education and transparency matter most in this equation. Anyone who cares about vegan and vegetarian standards can push brands to improve their labels, ask for certification, and create louder demand for clarity. Simple questions—“Is titanium citrate in this capsule derived without animal byproducts?”—encourage better labeling and stronger ethical standards.
| Names | |
| Preferred IUPAC name | tris(2-hydroxypropane-1,2,3-tricarboxylato)titanium(IV) |
| Other names |
Titanium(IV) citrate Titanium citrate complex |
| Pronunciation | /taɪˈteɪniəm ˈsɪtrət/ |
| Identifiers | |
| CAS Number | 89686-98-0 |
| Beilstein Reference | 3858723 |
| ChEBI | CHEBI:33222 |
| ChEMBL | CHEMBL61336 |
| ChemSpider | 16216940 |
| DrugBank | DB11251 |
| ECHA InfoCard | 03e1e3b2-58b0-4dcc-bb30-bf1d30455228 |
| EC Number | 263-657-4 |
| Gmelin Reference | 92077 |
| KEGG | C18672 |
| MeSH | D013972 |
| PubChem CID | 160955 |
| RTECS number | XI0350000 |
| UNII | T0J0445206 |
| UN number | UN3276 |
| Properties | |
| Chemical formula | C12H10O14Ti4 |
| Molar mass | 456.13 g/mol |
| Appearance | White or pale yellow powder |
| Odor | Odorless |
| Density | 0.8 g/cm³ |
| Solubility in water | Soluble in water |
| log P | -1.3 |
| Acidity (pKa) | 5.7 |
| Basicity (pKb) | 11.95 |
| Magnetic susceptibility (χ) | Paramagnetic |
| Refractive index (nD) | 1.54 |
| Viscosity | 500 - 1500 cP |
| Dipole moment | 3.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 311.8 J·mol⁻¹·K⁻¹ |
| Pharmacology | |
| ATC code | V03AX22 |
| Hazards | |
| Main hazards | May cause respiratory irritation. |
| GHS labelling | GHS labelling for Titanium Citrate: "GHS07, GHS08, Warning, H302, H332, H373 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. H335: May cause respiratory irritation. |
| Precautionary statements | Keep container tightly closed. Keep only in original container. Wash thoroughly after handling. Do not eat, drink or smoke when using this product. Wear protective gloves/eye protection/face protection. |
| Lethal dose or concentration | LD50 (Oral, Rat): > 2,000 mg/kg |
| LD50 (median dose) | > 12,000 mg/kg (rat, oral) |
| NIOSH | WF1350000 |
| PEL (Permissible) | 10 mg/m3 |
| REL (Recommended) | 75 mg/kg bw/day |
| IDLH (Immediate danger) | Not established |
| Related compounds | |
| Related compounds |
Ammonium ferric citrate Sodium ferric citrate |