The story of tri-n-butyl citrate winds through the evolution of plasticizers over the last century. From early days, factories and laboratories searched for less toxic, non-phthalate compounds to use in everything from toys to food packaging. Tri-n-butyl citrate entered that scene, offering a safer profile than many predecessors. During the boom years of plastics in the twentieth century, pressure from new safety regulations made the search for alternatives an urgent task. Researchers looking for molecules that could soften plastics without threatening health landed on citric acid derivatives like tri-n-butyl citrate. The food and pharma industries, especially in Europe and North America, quickly turned to this compound. Its acceptance grew as regulatory agencies paid closer attention to substances in contact with food or pharmaceuticals. Over time, changes in public awareness, combined with increasing reports on potential dangers of legacy plasticizers, kept tri-n-butyl citrate in the spotlight for chemists and manufacturers alike.
Tri-n-butyl citrate stands out as a colorless, nearly odorless oily liquid. Its role as a plasticizer, stabilizer, and solvent stretches across multiple industries. You’ll find it in dental materials, medical devices, food wraps, and tablet coatings. Makers value its ability to add flexibility without bringing toxicity. As societies get stricter on what goes into food contact materials and pharmaceuticals, the presence of tri-n-butyl citrate only keeps growing. The compound fits the modern push for safer materials—especially in products used by children or anyone sensitive to chemical leaching.
Tri-n-butyl citrate has a molecular formula of C18H32O7, weighing in at a molecular weight just above 360 g/mol. It shows up as a clear, slightly viscous fluid. Pointing to the facts, the liquid doesn’t mix well with water, but blends easily into most organic solvents. Its density sits near 1.05 g/cm³ and it carries a boiling point over 385°C, which means it stands up well in most manufacturing settings. It doesn’t freeze easily, usually holding its liquid state down below -40°C. The low vapor pressure means it won’t easily evaporate, an important trait for long-term goods like plastic packaging or medical tubes.
Any batch of tri-n-butyl citrate leaves the factory with a Certificate of Analysis. You’ll read that purity rests above 97 percent—often higher for pharmaceutical uses. Most suppliers supply detailed info about acidity, color (measured in APHA, usually below 50), and water content (almost always below 0.2 percent). Labels follow international standards for classification of chemicals, using hazard pictograms if needed, though this chemical usually avoids strict labeling thanks to its relatively low toxicity. Storage instructions tend toward cool, dry rooms away from strong acids or bases.
Factories manufacture tri-n-butyl citrate using a straightforward esterification process. They blend citric acid, usually derived from glucose fermentation, with an excess of n-butanol and add a catalyst, often an acid like sulfuric. A water-removal system promotes the completion of reaction and boosts yield. After enough time at elevated temperatures, the mixture gets purified by distillation and washing steps. This process requires careful temperature control and moisture monitoring, since too much water slows down the reaction and lowers quality. Over the years, improvements in catalyst choices and recycling butanol have cut down on production waste and energy consumption, making today’s plants more efficient.
Tri-n-butyl citrate holds up well under most typical conditions, showing strong resistance to hydrolysis under neutral or mildly acidic environments. The molecule can break down under heavier acid or base attack, especially over long periods—a reason why long-term storage stays away from harsh chemicals. Chemists have looked for ways to modify the compound, either by partial hydrolysis or transesterification, to tune the plasticizing effect or introduce new properties, but its basic structure remains widely favored for stability and predictability. While more complex derivatives exist, tri-n-butyl citrate’s popularity comes from its balanced chemical durability and cost-effectiveness.
Tri-n-butyl citrate goes by many names in the world marketplace. Some might call it tributyl citrate, TBC, or TB citrate. These variations show up on chemical supply lists, Material Safety Data Sheets, and shipping documents. The chemical registry number (CAS 77-94-1) stays consistent, helping buyers and inspectors sort through the maze of synonyms. Commercial producers brand their products with names that promise purity or suitability for food and pharma packaging. Pharmacopeias like USP or EP mention the compound under accepted nomenclature linked to citric acid esters, forming an important part of product traceability and compliance.
Tri-n-butyl citrate enjoys a reputation for safety that many plasticizers do not share. It carries a low order of toxicity, with few reports of harm from ordinary industrial or consumer use. The Food and Drug Administration in the United States, along with European agencies, green-light its use in food contact substances and pharmaceutical packaging. Facilities handling the compound follow standard chemical hygiene rules: gloves, eyewear, and decent ventilation. Workers require training in spill response and first aid, though the likelihood of harm remains small. The chemical avoids the lists tied to endocrine disruption and reproductive harm that have forced many competitors out of circulation.
Pharmaceutical companies line their tablet coatings with tri-n-butyl citrate to keep pills stable, tasty, and easier to swallow. Food manufacturers use it in the wrappers and films that touch everything from candy bars to processed cheeses. Medical kit makers depend on its flexibility in tubing, blood bags, and even bandage adhesives. The cosmetics sector finds it a valuable fix for adjusting the feel and application of certain formulations without bumping up the risk of skin reactions. Demand in adhesives, inks, and sealants continues to rise as environmental agencies limit the use of more hazardous chemical softeners. These broad application fields tell a story about a chemical that keeps adapting to new regulatory and consumer pressures.
Research keeps finding new wrinkles in how tri-n-butyl citrate can help different industries. Kitchens and food labs test its stability in high-temperature packaging methods like retort and microwave heating. Pharma techs analyze how it helps control the release of active ingredients in time-released pills. Material scientists at universities probe for new catalysts or “greener” synthesis routes that cut emissions, recycle solvents, or use biobased n-butanol. Clinical researchers want to know if tri-n-butyl citrate can further replace less safe plasticizers in pediatric or implantable medical devices. Across all sectors, pressure mounts for alternatives that check the boxes for low migration, low toxicity, and strong performance.
Researchers have put tri-n-butyl citrate through a long series of toxicological tests. Oral, dermal, and inhalation exposure studies reveal low acute toxicity. Animal models show high tolerance, with adverse effects showing up only at doses far above practical human exposures. Reproductive and developmental toxicity studies have failed to turn up major red flags, unlike many phthalate alternatives. That said, some metabolism studies probe possible breakdown into n-butanol and citric acid, both of which the body can manage at typical exposure levels. Regulatory fetch papers cite its negative listing for mutagenicity and carcinogenicity at the doses that matter for consumers. This risk profile allows governments and health watchdogs to focus concerns on much more hazardous plasticizers.
The future looks busy for tri-n-butyl citrate. Clean-label trends in food and medicine drive the search for ingredients with both a track record and scientific backing. As governments from India to the EU raise the bar on what goes into consumer products, expect more demand for non-phthalate plasticizers. The move toward biodegradable and compostable plastics pulls the industry toward safe, non-persistent additives—an area where tri-n-butyl citrate already fits the bill. Advanced composite materials, 3D printing filaments, and next-gen medical devices offer new playgrounds for the compound. Future research will likely produce tailored variants or improved production processes, but the foundation set by tri-n-butyl citrate looks strong, grounded in both regulatory trust and broad technical utility.
Tri-n-butyl citrate pops up in more products than most people might guess. Pull a soft plastic toy out of a package, squeeze it, and that flexibility often comes from plasticizers mixed in during manufacturing. Tri-n-butyl citrate is one of those softeners. It does this job well without leaving behind a strong odor or sticky mess. Long ago, chemists realized it stayed stable at higher temperatures, which matters for materials meant to hold up through heat and sunlight. Polyvinyl chloride (PVC) is a big customer. Without this kind of additive, PVC would crack and turn brittle over time.
One key place this chemical shows up is in packaging for food, coated papers, and wraps. Regulators have looked closely at its safety, with FDA green lights giving manufacturers confidence. Having worked in a food processing plant, I remember safety officers double-checking every plastic container—tri-n-butyl citrate always made the list of approved additives. This chemical does not taint the taste or smell of foods, which helps it hang on to its spot in production lines. In products where strict limits on chemical leaching apply, it stays popular, especially compared to older, riskier plasticizers.
Cosmetics companies appreciate chemicals that are non-irritating, light, and odorless. Tri-n-butyl citrate lands in nail polishes as a fixative, helping color stick and shine without fading. I've come across it on ingredient lists of skin creams and lotions too, where it works as a spreader, helping oils mix without feeling greasy. Given how easily customers react to ingredients, anything that blends in quietly and passes toxicology checks wins favor. Outside of skin contact, the chemical shows up in perfumes and fragrances, smoothing out the mixture without changing the scent profile.
Hospitals and clinics demand flexible plastics for bags, tubes, and gloves, but can’t risk toxic leaching. Tri-n-butyl citrate earns its place here. Having volunteered on a hospital supply chain panel, I saw how purchasing officers replace older phthalate plasticizers with this one. Reports from the World Health Organization and several environmental groups back up those choices. Blood bags, IV lines, and certain pill coatings all rely on plasticizers like this to strike a balance between flexibility and purity.
Even a safe track record raises questions. Some watchdogs point to possible buildup in groundwater near factories, so responsible disposal remains top of mind. Companies now keep a closer eye on recycling streams, and wastewater treatment gets regular reviews. Consumers want fewer chemicals lingering in the environment, so manufacturers test for breakdown rates and possible bioaccumulation. Pushing for more biodegradable alternatives could take the pressure off downstream.
Public pressure helps drive companies to share more about their supply chains and substitute newer, safer additives where possible. Open science, tighter rules on testing, and steady reviews from agencies like the FDA can all work together to protect families and factory workers alike. I’ve seen employee trainings get tougher, with chemical safety sheets now part of most onboarding checklists. Seeking out plastics and cosmetics with clear ingredient labels allows people to avoid what concerns them, making it easier to be informed about what goes on and in their bodies.
Tri-n-butyl citrate, a clear liquid, often finds its way into plastics to give them flexibility. Think of soft squeeze bottles or cling film. It’s made by bringing together citric acid and butyl alcohol, and some folks in the food business have shown interest in it as a plasticizer for packaging, especially when regular phthalates come with worries. But does that mean it’s safe for food contact or, more importantly, for any material that might leach into what we eat?
Regulatory scrutiny sets the bar for what we can trust around our food. The U.S. Food and Drug Administration (FDA) lists Tri-n-butyl citrate as an allowed indirect food additive. The agency places strict limits on how much can migrate into food. European authorities have also evaluated this compound through the European Food Safety Authority (EFSA). Their scientific panel studied available data, including toxicology studies in animals and measurements of how much might migrate from packaging into food. With this research, both the FDA and EFSA allow its use in various food contact applications, provided users observe maximum migration limits.
I’ve worked on projects with food manufacturers and packaging suppliers aiming to balance flexibility in plastics with health and safety. The question of chemical migration often comes up in meetings and training sessions. One plant manager once poured out his worries, recalling reports from years prior about compounds leaching out of packaging. His team wanted solutions rooted in science, not just “it should be fine” assurances. That experience made clear how much people in the food industry care about what ends up near our food.
Every additive or material has to clear a high bar. Tri-n-butyl citrate, compared to old-school phthalates, shows lower toxicity in animal studies. The main risks studied include reproductive effects, and current data doesn’t show the same concerns seen with phthalates. Still, nobody wants surprises. Regulators set migration thresholds based on safety study results. Analytical chemists test finished products, plastics, and migrated residue, ensuring the compound remains within safe bounds. If the use stays within the prescribed limit, scientific consensus today says it’s safe for food packaging.
Tri-n-butyl citrate carries approval for food packaging, not for direct use in food as a flavoring or preservative. Ingesting the substance directly tells a different story—animal testing shows high doses may cause digestive upset and other side effects, which stays a mile away from its trace presence in most packaging. Regulators haven’t cleared it for direct consumption because safety margins built for packaging migration do not translate to high-concentration exposure.
Most shoppers don’t need to worry about trace amounts migrating from packaging approved by trusted authorities. Looking for “BPA-free” or “phthalate-free” packaging sometimes means Tri-n-butyl citrate is in the mix, stepping in as the flexible friend. Consumers can stick to products where safety reviews have been done. If concerns remain, food fresh from glass or waxed paper offers peace of mind. For those on the inside of manufacturing, regular supplier audits and lab migration tests keep everyone on their toes, making sure only compliant materials touch the food we buy.
As debates around chemical safety evolve, open disclosure will keep public trust intact. No system works perfectly forever; fresh science and vigilant monitoring protect public health. Tri-n-butyl citrate draws less controversy than phthalates, but the public always deserves timely review and transparency. That blend—real oversight, clear science, and common sense—keeps food safety on track.
People often treat chemicals like Tri-n-butyl Citrate as low-risk because it features in products labeled “eco-friendly.” Yet safety at the warehouse and lab truly depends on not cutting corners. I’ve seen managers brush off labeling and container checks, usually because they think of this substance more as a green alternative than a real hazard. That’s not the mindset you want. Even a safer plasticizer spells trouble without everyday vigilance.
One thing about storage: temperature swings should never be left unchecked. Tri-n-butyl Citrate performs best at room temperature, away from direct sunlight and heat sources. Open drums near loading bays stack up risks during the hot season. I once watched a whole batch lose quality because drums sat in an unventilated shed in late summer. The plasticizer started showing discoloration and a funny smell—hard proof that sunlight and warmth can kickstart slow breakdown. Chemical suppliers say: stick between 15°C and 30°C, and always keep things dry. Humidity encourages hydrolysis and that causes by-products nobody wants leaching into what you’re making.
Ventilation gets overlooked for economic reasons, but it matters far more than many think. Tri-n-butyl Citrate vapor exposure rarely crosses acute levels, yet poorly vented warehouses can produce headaches, especially if you handle drums frequently or for hours on end. Good airflow cuts down risks and keeps odors from building up. Simple exhaust fans fixed a recurring air quality issue at a plant I once visited. After that, absenteeism from staff headaches dropped off sharply.
Storing this chemical looks straightforward: sealed drums, polyethylene or steel, kept upright on pallets. Plenty of folks reuse containers indiscriminately, which brings contamination. Once, a supplier sent a batch in drums that previously carried oils. The end product failed tests due to cross-contamination, costing days of rework. Dedicated, clearly labeled drums are non-negotiable. Mark the date and contents clearly—even hand-scrawled notes make a difference if labeling machines go down.
Spills seem like rare events, but even a small puddle of Tri-n-butyl Citrate on the floor can lead to slips and sustained exposure. I’ve cleaned spills before, and fast action with absorbent pads, followed by a thorough wash-down, avoids bigger problems. Keep kits close by, not locked in a back office. Employees should never scramble for basic tools in an emergency. Training counts more than paperwork here—people won’t panic if they know exactly where everything is.
Tri-n-butyl Citrate won’t explode like ether or corrode like hydrochloric acid, but that’s no excuse for slack procedures. A good routine short-circuits most mishaps: check seals daily, look for bulging or leaking drums, and always log each movement. Quality takes a direct dive when containers look battered or dirty, so regular inspections matter, even on busy days. The most reliable sites I’ve seen carry out safety walkthroughs before every shift and treat these routines as non-negotiable.
All these steps—the right temperature, dry storage, distinct containers, training for spills—stack up as basic respect for everyone working with Tri-n-butyl Citrate. Cutting corners might not show results immediately, but good habits pay off in worker health, product quality, and regulatory peace of mind. Treating this “green” component with the same seriousness as bigger risk chemicals signals real professionalism and delivers confidence to anyone downstream from the warehouse to the finished product.
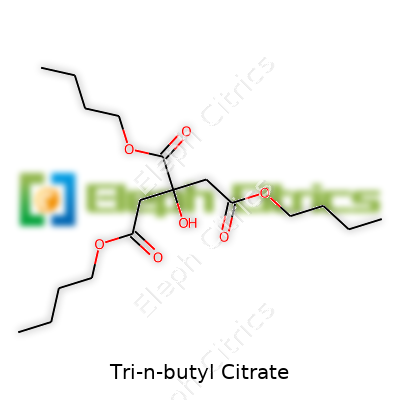
Tri-n-butyl citrate runs by the chemical formula C18H32O7. The backbone comes from citric acid, a familiar compound found in citrus fruits, but most people know it from labs and food preservation. Once you swap out the acidic hydrogen with three n-butyl groups, you get tri-n-butyl citrate. The structure shows three butyl chains branching off the core citric acid skeleton. It looks like a star with three long arms—each arm built from a butyl group, each linked with an ester bond.
To break it down more practically, the central part of tri-n-butyl citrate holds a small chain of carbons and oxygens—right where the action happens in citric acid. This core makes three strong connections to butyl groups, forming a stable, liquid molecule at room temperature. Bringing in those butyl chains changes how the molecule behaves: more oily, less reactive, and ready to blend into a whole field of plastics and coatings.
It's tempting to ignore these small details, but the shape and makeup of tri-n-butyl citrate drive everything it does. Those three flexible butyl arms add not just size but movement, and they don't let the molecule clump up in neat crystals. I remember seeing this consistency firsthand in a plastics facility—it gets poured out like syrup, not powder. That tells you a lot about how it blends in or softens other materials.
The ester bonds also matter. They're tough enough to last but react eventually under heat or acid, which is helpful for environmental breakdown. The molecule steers clear of the health risks tied to older plasticizers like phthalates. If you dig into the research, you’ll spot studies pointing out that alternatives like tri-n-butyl citrate do not release the same harmful compounds over time.
Tri-n-butyl citrate shows up in soft plastics, adhesive films, and even in some medical products. The need for safer additives has pushed its popularity higher. Phthalates—once dominant—keep turning up in headlines tied to health risks. I’ve seen this shift up close. Clients worried about consumer safety started asking for certifiable alternatives. Tri-n-butyl citrate gives manufacturers a tool for softening plastics without triggering the same safety alarms. It’s approved by regulatory agencies around the world, including in the U.S. and Europe, for specific food packaging and medical applications.
It even finds its way onto pills as a coating ingredient. Because the molecule stays flexible at a range of temperatures and resists migration out of plastic, it doesn't carry the downsides often seen in outdated plasticizers. This advantage offers more than peace of mind: less chemical leaching means less environmental cleanup.
Choosing tri-n-butyl citrate boils down to commitment—both to safety and to product performance. Companies switching over must study their supply chains, trial formulations, and look at costs. Yet with more data showing the long-term health and ecological benefits of cleaner plasticizers, change grows easier to justify. For those of us working with materials and product safety, knowing the structure and formula of tri-n-butyl citrate isn’t just technical trivia. It builds the case for smarter, safer chemical choices in everyday manufacturing.
Tri-n-butyl citrate, or TBC, isn’t a name many shoppers toss around, but lots of folks touch its effects without realizing. You’ll spot TBC as a plasticizer in products from food wraps to toys, even in cosmetics. It shows up whenever manufacturers want plastic to stay flexible and safe, especially where children and food come into play. Stepping away from phthalates, which grab headlines for their health risks, TBC looks like a safer swap at first glance.
Plenty of people ask if TBC actually breaks down after use. Lab tests say yes—TBC can degrade in environments rich with microbes. One study from the late ‘90s, widely cited in chemistry circles, found TBC biodegrades in soil and water under aerobic conditions. Another test, the OECD 301C, marked it as “readily biodegradable.” In wastewater treatment setups, over 90% of TBC broke down within weeks. These are encouraging numbers compared to the usual synthetic plasticizers, which linger for years.
Yet those lab results don’t always match what happens after a product lands in the trash or heads down a drain. Environmental conditions shift quickly—soil chemistry, climate, waste management practices all affect whether TBC actually vanishes without a trace. Some field data suggests that colder climates, low-oxygen settings, or heavy-duty landfills slow decomposition, letting TBC stick around far longer.
TBC’s promise boils down to trading off the well-documented dangers of phthalates for a molecule that doesn’t mess with hormones or build up in the body as easily. So far, toxicology reports paint a reassuring picture: TBC doesn’t disrupt endocrine systems the way DEHP or DBP do. European and North American regulators cleared TBC for use with foods and in toys, though they keep an eye on it as more real-world data trickles in.
It’s not a green light to use and forget. Even a less bioaccumulative plasticizer still puts pressure on waste streams. Researchers highlight that in waterlogged fields or poorly ventilated landfill layers, some of TBC survives and might nudge aquatic life or groundwater in ways nobody intended. This is an experience we know from dozens of “once-safe” chemicals—environmental impact often sneaks up over years rather than months.
For companies and consumers who care where their stuff ends up, TBC represents a step forward, though not the finish line. Choosing a more biodegradable plasticizer matters, but tracking exactly how and where our trash degrades stays the tougher job. Some waste management outfits already explore specialized composting or recovery streams for these kinds of additives. Stepping up public investment in real-world biodegradability testing could tighten the gap between lab optimism and field performance.
On a practical level, a little knowledge goes a long way. Asking manufacturers if their materials use TBC, or pushing for certified “compostable” packaging, gives consumers a shot at steering demand. If family or friends ask about greener plastics, it makes sense to say TBC stands out compared to legacy chemicals, but that tossing stuff carelessly doesn’t guarantee it simply fades away. People who keep pressure on producers—through questions and votes at the checkout—help shape a market that values not just low hazard, but real traceability and transparency.
The search for truly environmentally friendly chemicals is a moving target. Using TBC means taking a better shot, not hitting the bullseye just yet.
| Names | |
| Preferred IUPAC name | 2-hydroxypropane-1,2,3-triyl tributanoate |
| Other names |
Butanoic acid, 3-hydroxy-, tributyl ester Citric acid tributyl ester Tributyl 2-hydroxy-1,2,3-propanetricarboxylate TBC Tributyl citrate |
| Pronunciation | /traɪ n ˈbjuːtɪl ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 77-94-1 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Tri-n-butyl Citrate**: ``` C(CCOC(=O)C(CCOC(=O)C(CCOC(=O)OCCCC)O)O)COCCCC ``` This is the **SMILES** string, which can be used to generate the 3D JSmol model. |
| Beilstein Reference | 740214 |
| ChEBI | CHEBI:34779 |
| ChEMBL | CHEMBL1401969 |
| ChemSpider | 53301 |
| DrugBank | DB11255 |
| ECHA InfoCard | ECHA InfoCard: 03-2119726365-41-0000 |
| EC Number | 205-775-0 |
| Gmelin Reference | 2043663 |
| KEGG | C18603 |
| MeSH | D017987 |
| PubChem CID | 60961 |
| RTECS number | **TSJ6.6827** |
| UNII | 5J1P2U8M92 |
| UN number | UN3077 |
| Properties | |
| Chemical formula | C18H32O7 |
| Molar mass | 402.54 g/mol |
| Appearance | Colorless transparent liquid |
| Odor | Odorless |
| Density | 1.05 g/cm³ |
| Solubility in water | Insoluble |
| log P | 1.95 |
| Vapor pressure | 0.2 mmHg (130°C) |
| Acidity (pKa) | 5.4 |
| Basicity (pKb) | pKb: 3.20 |
| Magnetic susceptibility (χ) | -7.92e-6 cm³/mol |
| Refractive index (nD) | 1.443 |
| Viscosity | 25-32 mPa·s (20°C) |
| Dipole moment | 2.3 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 579.6 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1167.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4461.7 kJ/mol |
| Pharmacology | |
| ATC code | A07BC06 |
| Hazards | |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07, GHS08 |
| Signal word | Warning |
| Hazard statements | H315: Causes skin irritation. H319: Causes serious eye irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378 |
| NFPA 704 (fire diamond) | 1-1-0-0 |
| Flash point | 165 °C |
| Autoignition temperature | 355°C |
| Explosive limits | Explosive limits: 0.6%–8.2% |
| Lethal dose or concentration | LD50 Oral Rat > 5000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 = 30,000 mg/kg |
| NIOSH | TIJ40 |
| PEL (Permissible) | Not Established |
| REL (Recommended) | 250 mg/L |
| Related compounds | |
| Related compounds |
Tributyl acetylcitrate Acetyl tributyl citrate Triethyl citrate Triphenyl citrate Trimethyl citrate |