Triethyl citrate entered the scene in the early twentieth century, fueled by the need for better plasticizers. Chemists once leaned heavily on phthalates and other esters but soon ran into concerns about toxicity and plastic degradation. The focus shifted to citrates, and triethyl citrate quickly earned trust for its lower toxicity and renewable origins. This compound’s history reflects a learning curve common throughout industrial chemistry, where public health follows just a step behind commercial innovation. The food, pharma, and cosmetics industries pushed for safer options, and triethyl citrate found its footing fast. Constant changes in regulatory attitudes ensured production processes matured alongside consumer protection demands, so this story isn’t just about invention but also about adapting to the needs of workers and consumers.
Triethyl citrate isn’t some boutique chemical with niche utility. It’s an ester obtained by combining citric acid with ethanol. People see it show up as a stabilizer for flavors, a plasticizer for plastics and coatings, and even as an excipient in pills and capsules. Companies choose it not just for technical reasons but because the alternatives have raised too many questions over the years. The move toward bio-based chemicals has added to its appeal, allowing manufacturers to market finished goods as safer or “greener.” Triethyl citrate is clear, colorless, and carries only a faint smell. Applications aren’t tied to one industry, and the range spans from candy coatings to nail polish removers and deodorant sprays.
Triethyl citrate appears as a clear, colorless liquid that mixes smoothly with alcohol and various organic solvents. Water solubility is low but not negligible, offering some versatility. It holds a molecular formula of C12H20O7 with a molecular weight hovering around 276.28 g/mol. Boiling point hangs in the ballpark of 294°C, and this high tolerance pays off in applications demanding heat resistance. Viscosity is moderate, sitting between greasy and watery, which makes blending with other liquids straightforward. Its vapor pressure stays well below risky thresholds at room temperature, cutting down on inhalation hazards in the manufacturing process.
For technical specs, most suppliers stick to tight purity standards—usually over 99% purity by weight. Color metrics like APHA barely register when quality is high, and acid value typically lands well below one. Major suppliers conduct gas chromatography examinations, making sure impurities like diethyl citrate or ethanol sit far below regulatory limits. Labeling in Europe falls under E1505 for food-grade applications. Pharmaceutical labeling relies on the name “triethyl citrate” and includes warnings about storage, handling, and suitability for different delivery methods. Companies shipping to the U.S. name it under the Food Chemicals Codex, which guarantees transparency for end-users.
Factories churn out triethyl citrate by direct esterification of citric acid and ethanol, typically with mineral acids—often sulfuric acid—as catalysts. Operators conduct the process under reflux to boost yield, removing water as it forms to push the reaction forward. After the initial conversion, purification steps like vacuum distillation and neutralization follow. Any trace mineral acid or side-products exit before packaging. Chromatographic monitoring tracks process effectiveness and purity, so large-scale production relies as much on tight process control as on good raw material selection. The shift toward using bioethanol provides an added benefit, especially for suppliers chasing sustainability points.
Most chemical interest in triethyl citrate boils down to its ester groups. Scientists tweak those groups for modified characteristics, or hydrolyze them to break the molecule down again into citric acid. This flexibility sets triethyl citrate apart from more rigid plasticizers. In mixed reaction pathways, it can act as a backbone for more elaborate esters, and its mild reactivity in acidic or basic environments prevents unwanted breakdowns during end-use. Some polymer chemists look at chain-end modifications, grafting onto triethyl citrate to adjust flexibility or adhesion in specialty coatings. Cross-linking with certain alcohols or acids generates copolymers that find their way into high-value electronics or pharmaceutical coatings.
In the marketplace and lab, this material appears under several banners. Beyond “triethyl citrate,” folks call it Citric acid, triethyl ester; Ethyl citrate; or TEC. European food regulatory codes list it as E1505. Some manufacturers trademark blend names that include triethyl citrate, especially in cosmetic or personal care applications. Pharmaceutical excipient catalogs stick to the proper chemical name, though “TEC” gets tossed around in everyday factory dialogue. When labels mention solvents or stabilizers, spotting “triethyl citrate” among ingredients has become much more common over the last decade as people shy away from legacy phthalates.
First-hand shop floor experience with triethyl citrate stands in contrast to handling more hazardous esters. The compound poses low acute toxicity, yet best practices still dictate gloves, goggles, and adequate ventilation. It rarely triggers skin or eye reactions, and most regulatory bodies set no lower occupational exposure limits beyond general organic solvent rules. As with any ester, overheating during production or use can decompose it, so teams monitor temperature and avoid open flames. In terms of environmental management, triethyl citrate biodegrades quickly in most environmental systems, so it does not pile up or worsen aquatic toxicity. Handling practices look similar across pharma and food companies, driven by shared quality audit requirements.
Triethyl citrate finds its way into candy coatings, where it helps bind flavors and stabilize textures. In pharmaceuticals, it prevents films from cracking during tablet pressing or storage. In plastics, especially for kids’ toys and medical devices, it stands out where phthalates face bans. I’ve watched manufacturers switch over old lines from DEHP or DBP to triethyl citrate to meet European REACH guidelines. Personal care companies use it heavily in deodorants, acting as a fixative that doesn’t interact with active ingredients or fragrances. Many powder processes benefit from it as an anti-caking agent, ensuring shelf-stable, lump-free products.
The research landscape for triethyl citrate keeps shifting. Some groups investigate it as a carrier for poorly soluble drugs, reasoning that its mild reactivity and non-toxic metabolism give it an edge. Others work on using it in biodegradable plastics, especially where food contact rules block the use of uncontrolled plasticizers. Academic labs investigate new uses in nanoparticle delivery, targeting improved safety in inhaled or injected medications. On the industrial side, engineers tweak reaction parameters to reduce waste, boost yields, and replace petrochemical feedstocks with sustainable plant inputs. The march toward circular chemistry places triethyl citrate front and center as a model for esters with cleaner life cycles.
Compared with many plasticizers, triethyl citrate scores well for toxicity. Animal study data shows no developmental or reproductive impacts at realistic exposure levels. Metabolism spits out harmless breakdown products—mostly citric acid and ethanol, both rapidly used up by the body. Food safety authorities have assessed its use in large-scale ingestion, confirming safety even for sensitive sub-populations. Many manufacturers still run batch-by-batch impurity checks, since byproducts or accidental contamination can alter toxicity profiles in unexpected ways. Occupational exposure studies back up its low-risk reputation, but regulators maintain a watchful stance as new research comes in, especially in long-term food or pharmaceutical studies.
Triethyl citrate isn’t likely to fade away anytime soon. Growing restrictions on synthetic plasticizers, along with heightened consumer concern over chemical safety, keep driving demand. Producers with integrated bio-based operations have begun pushing triethyl citrate to the forefront, using its safe reputation to build supply chains that look both profitable and ethical. Researchers tinker with structure-activity relationships, hoping to eke out better performance in coatings, drug delivery, and food preservation. Downstream industries—especially those with tight pollution controls—turn to triethyl citrate as a way forward that dodges the worst regulations and headlines tied to phthalates or other esters. As more countries hammer out tougher safety standards, the value of a well-understood, low-toxicity chemical only strengthens. Manufacturers find themselves relying more on experience and data—two resources that build real trust between producers, regulators, and users.
Triethyl citrate sits in a tricky spot — it barely gets noticed by most people, yet it gets added to plenty of everyday products. This chemical starts out as an ester of citric acid, created through a process involving ethanol. Once produced, it shows up everywhere: in foods, cosmetics, medicines, and even e-cigarettes. Triethyl citrate earned its spot on ingredient lists because it’s non-toxic, biodegradable, and gets along well with other ingredients. These qualities mean many companies trust it enough to use where safety matters most.
Looking at food, triethyl citrate keeps things from separating. I’ve seen it labeled as an “emulsifier” on countless soft drink and candy packages. Those clear sodas and bright gummies don’t just look fresh by accident — this ingredient helps keep oils and other flavors from turning cloudy or spoiling. In baking powders, it acts as a stabilizer, making sure ingredients don’t break down before they should. Over the years, the FDA said it’s safe as a food additive, so consumers eating processed foods get a tiny dose of it from time to time without thinking twice.
Pharmaceutical tablets need to dissolve at the right place and right time, especially for medicines with sensitive coatings that protect the stomach or time-release ingredients inside the body. Triethyl citrate plays a behind-the-scenes role, giving pills a smooth coat that resists chipping during transport but dissolves as intended inside the body. It also prevents moisture damage while the pills sit on shelves across pharmacies or medicine cabinets. Plenty of generic pillmakers rely on it, since it mixes well and doesn’t irritate digestive systems.
Consumers who check labels on deodorants or hairspray spot triethyl citrate often. It’s added to deodorants because it stops sweat from smelling, basically by reducing the bacteria that turn sweat into odor. I’ve seen some natural brands highlight it since it doesn’t block pores, unlike some old-school aluminum-based compounds. In hair sprays and nail polish removers, it acts as a plasticizer — keeping things flexible but preventing clumps or cracks. These roles matter, especially now that shoppers demand functional ingredients and safety, all wrapped into one.
While some additives raise health alarms, few studies link triethyl citrate with allergies or toxicity. The compound breaks down into citric acid and ethanol in the body, both substances our livers handle from other foods or drinks. Still, researchers continue to examine all additives for long-term effects — especially since exposure can add up over time, even at low individual doses. Stronger regulation, more transparent labeling, and continued third-party testing help consumers build trust in what goes into their bodies.
Consumers want safer, eco-friendly options, whether shopping for snacks or skin care. Triethyl citrate deserves its reputation for safety and versatility — but as new technologies arrive and demand for transparency grows, companies face continued pressure to keep reviewing how and why they use every ingredient. The challenge: build products people trust, based on solid, current science and honest communication. Triethyl citrate, subtle as it is, shows the power of these small choices hiding in the ingredients list.
Triethyl citrate shows up on food ingredient lists, lipstick labels, and even some medicines. It comes from citric acid, which also gives lemons their sourness. In food, companies use it to keep flavors stable, especially in things like candy and drinks. Some athletes have swallowed capsules of this stuff as an alternative to other food additives. You’ll probably find it listed as E1505 if you scan the back of European products.
People ask whether this ingredient stacks up well for health. Back in 1990, the Joint FAO/WHO Expert Committee on Food Additives decided triethyl citrate’s effects don’t raise alarms. Their scientists combed through animal data using large doses, a common starting point for risk checkups. They noticed you’d have to chow down hundreds of milligrams for every kilogram of body weight — every day — to see any possible side effects in their tests. Most folks get nowhere near those amounts in a modern diet.
The European Food Safety Authority took another look just a few years ago. Their panel concluded that consuming typical amounts from food and drinks stays far below levels that cause problems. They recognized some breakdown happens in the belly, making less risky citric acid and a little bit of alcohol, both already familiar to the human body. E1505 doesn’t linger in blood or store up in your tissues, so it leaves the body quickly.
Having worked in kitchens, I’ve seen food colorings and preservatives go in and out of favor. I remember worrying as a kid about scary ingredient names, until I saw my grandmother toss hand-squeezed lemon juice over fish for flavor. Triethyl citrate may sound like some wild chemical, but in practice, it’s turned into water-soluble pieces your liver already handles daily.
There are real stories behind ingredient safety. Allergic reactions, stomach trouble, and other complications often pop up with new additions, but triethyl citrate doesn’t seem to do that based on decades of use. It acts more like a background player, letting other flavors stand out. A glass of soda, a stick of gum, or a baked treat probably uses this compound in such tiny doses, it doesn’t stand out any more than citric acid itself.
I get why people side-eye unknown additives, especially after reading about corporate slip-ups. The urge to scrutinize every label is common sense today. Anyone with allergies or a rare health issue should talk with a doctor before introducing new manufactured ingredients, just to be safe. This fits with advice from government agencies and nutrition experts around the world.
The best approach, from what nutritionists say and my own experience shopping for my family, is this: focus on the whole food, not just one additive. Fresh fruit, home-cooked meals, and less processed stuff mean lower exposure to ingredients you question. Food scientists continue reviewing each additive, including triethyl citrate, so folks can make choices based on updated science, not outdated fear.
Food safety rests on review and openness. If an ingredient needs closer study, regulators and researchers should share their findings with the public in plain language. That builds trust, not just in labels, but in the whole food system.
People look at ingredient lists with different expectations. I remember flipping over bottles in the supermarket, trying to decode words like “triethyl citrate.” The question keeps coming up: is this stuff natural, or synthetic? In simple terms, triethyl citrate starts with citric acid, a substance in lemons, limes, and many fruits. Through a chemical process called esterification, citric acid combines with ethanol. The ethanol used in manufacturing typically comes from grains through fermentation. Now, there’s a catch. None of this takes place inside a fruit or vegetable; it’s done in a factory. Even though you could say the starting materials come from nature, the final product gets made in a lab.
Labeling rules leave gaps. I have spoken to folks who see “derived from plants” and feel misled after learning more. People care if something is natural because it often feels safer or healthier. For triethyl citrate, regulators such as the FDA give it a green light as a food additive and consider it generally recognized as safe (GRAS). Food and cosmetic companies rely on this status, using it to keep baked goods fresher longer or help perfumes last. Still, this doesn’t answer what “natural” really means for most shoppers.
The confusion comes from marketing language. The US and EU often call something “natural” if the ingredients started out from plants, even if manufactured using industrial chemistry. Producers often market triethyl citrate as nature-based, but the reality is, nobody extracts it directly from oranges or lemons with a squeeze. This matters most for people with allergies, those concerned about chemical exposure, and folks trying to live sustainably. I’ve noticed that people on forums and at farmer’s markets often feel tricked when they learn how much processing goes into many so-called ‘natural’ additives.
I’m not against synthetic compounds, especially with data showing triethyl citrate serves as a safe alternative to harsher chemicals in deodorants and as a stabilizer in foods. Still, here’s the thing: if people want a truly “natural” ingredient, triethyl citrate doesn’t pass that test. Only transparency helps bridge that gap. The more people know how an ingredient is made, the easier it becomes for them to make informed choices.
Transparency starts with honest labels. Ingredient facts panels should go beyond vague language like “plant-based” and “naturally derived.” Shoppers deserve clear language: triethyl citrate is synthesized from citric acid and ethanol—both plant-based, but combined industrially. That information won’t scare off everyone, but gives the choice back to the consumer.
Another issue is sustainability. If a chemical comes from a renewable source, it helps shrink our reliance on petroleum-based goods. The raw materials in triethyl citrate support a smaller carbon footprint than some synthetic alternatives. That said, sustainability must go hand-in-hand with full disclosure of the chemical process, including the energy used and waste generated.
It makes sense for companies and regulators to standardize language around “natural” and “synthetic.” I’ve seen other industries, such as organic farming, build trust by setting clear definitions and third-party certification. Food and cosmetics can do the same. Open conversation and more education help close the gap between industry shorthand and what shoppers want.
Triethyl citrate often slips under the radar. Most folks rub on deodorant, chew gum, or swallow pills without worrying about every ingredient. This compound steps in as a mild plasticizer, a flavor fixative, and even a way to keep sticky hands at bay in pharmaceuticals and food. The FDA labels it "generally recognized as safe" (GRAS) when used within approved limits. Problems rarely come up, but rare doesn’t mean impossible.
Most people tolerate triethyl citrate without trouble. A small number might spot skin irritation in products like roll-on deodorant or cosmetics that rest on the skin for hours. The symptoms look like redness, a little itching, or a light rash. Unlike some stronger chemicals, triethyl citrate rarely fuels big allergic reactions or burning, but sensitive skin can still grumble about new formulas.
Folks working with pure triethyl citrate or breathing in its fumes during manufacturing might feel headaches or a scratchy throat. Strong smells and chemical handling can knock the wind out of anyone, so a mask and solid ventilation system pay off in jobs where contact runs high. Still, users in real-world situations—applying it on the skin or eating trace amounts in candy—come up safe almost every time.
Triethyl citrate moves through the digestive tract without much fuss. It turns into citric acid and ethanol as enzymes break it down. These byproducts already swim in fruit juice or wine at much higher levels. The body knows what to do with them, pulling in the citric acid and filtering out ethanol. Someone gobbling large amounts far beyond what the FDA says is safe could run into loose stools or a belly ache, but this remains vanishingly rare since food and drug regulations put tight caps on dosage.
Stories crop up about “safe” chemicals leading to chronic complaints for some folks. A reaction that seems mild for most could run rough for anyone with allergies, compromised skin barriers, or unique metabolic quirks. Documented incidents of harm from triethyl citrate come in thin, but keeping tabs on these outlier cases matters for public trust and ongoing safety research. Emergency rooms seldom see problems from this chemical, but labels should stay clear, and people deserve access to support if they run into trouble.
Clear communication between manufacturers, doctors, and consumers makes the difference. Labels should state possible irritants plainly, not buried behind Latin names or in fine print. Dermatologists often nudge people toward patch tests if new irritation pops up. At the workplace, updated training and better ventilation are good calls for anyone handling chemicals at scale.
Regulations already keep triethyl citrate contained, but new uses in health products—or in new combinations—mean continued safety monitoring holds value. Reporting skin reactions or gastrointestinal problems doesn’t just help the complainer; it sharpens the big picture for regulators who review GRAS status yearly. Everyone who eats, sprays, or handles these products has a role in watching for side effects and keeping the record up to date. Trust grows when people see that checks and honest conversation don’t stop once a chemical lands on a “safe” list.
Every day, people lather on lotions, creams, sprays, and other personal care products—rarely pausing to wonder about ingredients like triethyl citrate. This compound usually shows up in the fine print, lumped among a slew of chemicals. It's no secret that safety and transparency matter in today’s personal care industry. People want to know what goes onto their skin and into their bathrooms—and why it matters.
Triethyl citrate plays a role as a solvent and a fragrance fixer. Some deodorants rely on it for its ability to slow down the breakdown of sweat, helping keep underarms fresh without blocking pores. The molecule itself is an ester of citric acid, which you’d find in citrus fruits. Manufacturers gravitate toward it because it’s colorless, odorless, and doesn’t irritate most skin.
Across Europe, regulators scrutinize cosmetic ingredients with more rigor than almost anywhere else. Health agencies there allow triethyl citrate in cosmetics without major restrictions—so long as manufacturers follow concentration limits and quality standards. The European Chemicals Agency concludes that the compound breaks down safely in the body into ethanol and citric acid, both of which occur naturally. In the US, the Food and Drug Administration marks it as generally recognized as safe (GRAS), especially when used as a food additive.
Years ago, searching for an aluminum-free deodorant, I stumbled upon a brand highlighting triethyl citrate as a “science-backed alternative.” After months of use, there was never a hint of skin irritation—and no stains or strange scents. Digging into scientific research later, I found clinical studies backing up what I’d noticed. Dermatological journals don’t list many concerns tied to its topical use, even for people with sensitive skin. It works gently, especially compared to harsher preservatives or synthetic fragrance stabilizers.
Though triethyl citrate scores high on safety, transparency stays critical. Ingredient lists are often a jungle for average shoppers. Honest labeling helps people with allergies, or those driven by values like veganism or environmental responsibility, make informed choices. This chemical isn’t commonly linked to allergies or toxicity, but it makes sense for brands to disclose everything upfront. As a parent, I always check for full ingredient lists on kids’ shampoos and body washes, steering clear of anything I can’t pronounce without a science background.
As sustainability takes center stage, consumers push brands to reduce their environmental impact. Triethyl citrate isn’t sourced from fossil fuels—it’s manufactured from citric acid, which companies often obtain through fermentation, not mining or oil refining. It biodegrades easily, so it doesn’t build up in waterways or soil. Still, some feel weary around anything that sounds synthetic. Beauty brands stand to gain by explaining these origins and environmental perks. Green chemistry helps bridge the gap between science and nature, giving people cleaner, safer products without the harshness of old-school ingredients.
Triethyl citrate has carved out a spot in modern cosmetic formulas. Science backs its safety for both skin and the planet. As someone who values both effectiveness and ingredient integrity, I look for companies that publish data, answer customer questions, and use ingredients that respect people and ecosystems. That kind of openness builds trust, which matters more than any marketing claim.
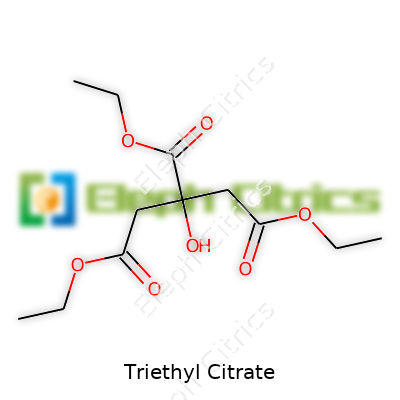
| Names | |
| Preferred IUPAC name | Triethyl 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Citric acid, triethyl ester TEC Trielin citrate 1,2,3-Propanetricarboxylic acid, 2-hydroxy-, triethyl ester Ethyl citrate |
| Pronunciation | /traɪˌiːθəl ˈsɪtreɪt/ |
| Identifiers | |
| CAS Number | 77-93-0 |
| Beilstein Reference | 1721751 |
| ChEBI | CHEBI:4884 |
| ChEMBL | CHEMBL14336 |
| ChemSpider | 5464 |
| DrugBank | DB13919 |
| ECHA InfoCard | 03e8e7c7-45da-42d0-b7a8-99f33cbad6a2 |
| EC Number | 204-823-8 |
| Gmelin Reference | Gmelin Reference: **83678** |
| KEGG | C14386 |
| MeSH | D013997 |
| PubChem CID | 6507 |
| RTECS number | GE8985000 |
| UNII | K8J7Q1N1MY |
| UN number | UN1981 |
| Properties | |
| Chemical formula | C12H20O7 |
| Molar mass | 276.28 g/mol |
| Appearance | Colorless oily liquid |
| Odor | Odorless |
| Density | 1.135 g/cm³ |
| Solubility in water | Soluble |
| log P | 0.12 |
| Vapor pressure | 0.00015 mmHg (25°C) |
| Acidity (pKa) | 5.4 |
| Basicity (pKb) | 10.13 |
| Magnetic susceptibility (χ) | -6.12 × 10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.439 |
| Viscosity | 16.6 mPa·s (20 °C) |
| Dipole moment | 3.52 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 348.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1166.1 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4041.7 kJ/mol |
| Pharmacology | |
| ATC code | A16AX19 |
| Hazards | |
| GHS labelling | GHS07 |
| Pictograms | GHS02,GHS07 |
| Signal word | Warning |
| Hazard statements | Hazard statements: "Causes serious eye irritation. |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P271, P280, P303+P361+P353, P305+P351+P338, P337+P313, P370+P378, P403+P235, P501 |
| NFPA 704 (fire diamond) | 1-1-0-0 |
| Flash point | 99°C |
| Autoignition temperature | 210 °C |
| Lethal dose or concentration | LD50 (oral, rat): 7000 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral, rat: 20,800 mg/kg |
| NIOSH | TIQ |
| PEL (Permissible) | PEL (Permissible Exposure Limit) for Triethyl Citrate: Not established |
| REL (Recommended) | 100 mg/kg bw |
| Related compounds | |
| Related compounds |
Citric acid Triethyl orthoformate Diethyl citrate Monoethyl citrate Acetyl tributyl citrate |