A century ago, chemists searching for safe alternatives to natural extracts turned their focus to citric acid derivatives. Citric acid itself had changed everything, bringing sour flavor and preservation power to a range of foods. Trimethyl citrate appeared on the scene as a result of these innovations. By the time the 1950s arrived, industries started seeing value in this colorless, slightly sweet-smelling substance. It emerged as more than just another chemical—its flexibility rendered it suitable for food, pharmaceuticals, and even flexible plastics. What started as a clever synthesis from citric acid and methanol grew into a mainstay for people seeking alternatives to phthalates or wanting less volatile solvents. Throughout the decades, technical improvements streamlined its synthesis, and regulatory bodies devoted more attention to its safety in consumer products.
Trimethyl citrate serves as a multifunctional ester derived from citric acid. Most commonly, it steps into roles that need mild plasticity without bringing toxic or environmentally persistent residues. Its slightly fruity odor works well for food contact applications. Manufacturers value it because it integrates well with both hydrophilic and lipophilic substances, making it useful across cosmetics, medical devices, and edible products. Many of these benefits rest on its unique molecular structure, which combines three methyl groups and a central citric backbone. Whether acting as a carrier for flavors or improving the feel of pills and gels, its utility stays clear across product ranges.
Physical form matters in the real world, not just on paper. Trimethyl citrate is a clear liquid with low volatility at room temperature. Its melting point swings around -16 degrees Celsius and it barely boils above 160-180 degrees Celsius under reduced pressure. Its water solubility sets it apart from rivals—unlike long-chain phthalates, this ester blends easily with water, ethanol, and many organic solvents. Viscosity often sits within manageable ranges, enabling easy dosing or mixing for manufacturers. Its refractive index, density, and moderate vapor pressure help chemists fit it into diverse applications without much adjustment in process equipment.
Industrial buyers scrutinize product certificates, so trimethyl citrate always comes with detailed specification sheets. These sheets provide CAS number, molecular formula (C9H14O7), purity (often above 99%), acid value, water content, and ester content. Heavy metal residues, color, and odor ratings also appear—especially for food or pharma grades. Labeling regulations push suppliers to display all known synonyms and hazard codes. Any residual methanol receives extra attention due to toxicity concerns. In the European Union and North America, labeling aligns with REACH, FDA, and international GHS standards, providing traceability from batch to batch. Compliance audits dive deep into these specifications because mislabeling can bring import rejections or product recalls.
The classic route for making trimethyl citrate involves direct esterification of citric acid with methanol. Strong acid catalysts, usually sulfuric acid, drive the reaction, with care needed to control temperature and water removal, since water formation slows conversion. In the laboratory, careful progression through condensation and distillation steps delivers the pure ester while minimizing unwanted byproducts. Industrial setups recycle methanol and control reaction rates to hit the best cost-versus-yield balance. Catalysts can alter the process timeline, but they also impact cleanup downstream. Eco-minded manufacturers are always on the lookout for new catalyst systems with lower environmental footprints or reduced waste generation.
Even though it has a stable ester structure, trimethyl citrate stands ready for more chemistry. It can handle mild saponification, splitting back to citric acid and methanol under basic conditions. Other reactions tweak its backbone for specialized uses—transesterification for making new esters, selective reduction for fragrance manufacture, or even mild oxidation for new polymer precursors. Cross-linked derivatives show promise in medical hydrogels and food packaging films. Chemists mining value from citric esters keep pushing for reactions that open the molecule to new uses without cranking up toxicity or cost.
Trimethyl citrate hides behind several names, which can lead to confusion unless buyers check labeling closely. Synonyms include “citrate acid trimethyl ester,” “methyl citrate,” and in code systems, “EEC No. E1505.” Some catalogs refer to it as “methyl 2-hydroxypropanetricarboxylate.” These names reflect its origins and structure but rarely appear in consumer-facing products. Industry players like to brand high-purity grades with their own marks and hold registrations across several languages for compliance. Chemists and quality assurance staff keep track of these synonym lists to avoid regulatory snags or supply-chain chaos.
Safety teams don’t take shortcuts with esters, considering exposure can sometimes lead to health surprises. Trimethyl citrate stands out as one of the safer options, earning low oral and dermal toxicity rankings in multiple regulatory reports. In the workplace, normal chemical handling rules apply—use of gloves, splash goggles, and proper ventilation. Its low vapor pressure reduces inhalation concerns, but methanol traces require special attention since methanol exposure causes nerve damage and blindness. Regulatory standards demand regular monitoring and strict batch documentation for pharmaceutical and food uses. Good manufacturing practice (GMP) and HACCP protocols lock in these protections, since recalls hurt both reputation and bottom line.
A walk through a modern grocery or pharmacy brings evidence of trimethyl citrate’s wide reach. Food technologists pick it for flavor stabilization and as an anti-caking agent in powdered mixes. Pharmaceutical formulators find it softens gelatin capsules and improves tablet consistency. Medical device manufacturers adopt it in coatings that need low toxicity yet effective barrier properties. The cosmetics industry turns to trimethyl citrate for skin creams, lotions, and fragrances, seeking non-greasy texture and good solubility. Some specialized plastics rely on it as a primary plasticizer—key for packaging sectors seeking alternatives to harsh phthalates. Increased scrutiny on endocrine disruptors in consumer goods means more companies look for proven, less hazardous substitutes like trimethyl citrate.
Trends in research show growing appetite for sustainable chemical feedstocks. Recent university studies peel back each part of the trimethyl citrate production process, aiming to swap fossil-based methanol with options like bio-methanol or green hydrogen-based routes. Analytical chemists also chase better detection and quantification in finished products, ensuring compliance remains watertight. In the polymer sector, innovators step up experiments on biodegradable films based on citric esters, searching for combinations that can rival conventional petrochemical plastics. Collaborative partnerships between food manufacturers and chemical suppliers have pushed development of custom blends that address shelf life, texture, or compatibility with other food additives. Ongoing research into functional foods may see trimethyl citrate play roles beyond just technical support—possibly even direct bioactivity.
No one wants a replay of past chemical safety scandals, so toxicology keeps a spotlight on trimethyl citrate. Studies involving oral, dermal, and inhalation routes consistently show low acute toxicity. Long-term studies in animals look for carcinogenicity and reproductive hazards; so far, evidence remains favorable relative to many synthetic plasticizers. European and US agencies keep a running evaluation to update risk profiles as new data comes in. Cell line assays and environmental fate modeling help round out the picture. Yet, caution persists, since unknown metabolite formation under certain uses or in vulnerable populations like infants or pregnant women can spark re-examination of accepted daily intake levels.
People want safer, greener, and more transparent ingredients in everyday products, and manufacturers watch regulatory winds closely. Trimethyl citrate checks several boxes: biodegradability, adaptability, broad safety margin, and ready supply from renewable resources. Ongoing innovation could see expanded roles in bioplastics, medical delivery systems, and even advanced food engineering. Efficiency gains in its manufacture could further shrink cost premiums over traditional plasticizers or solvents. If the chemical industry stays responsive to consumer and environmental needs, trimethyl citrate stands to gain a bigger slice of industrial and consumer markets—helping move products away from persistent, potentially hazardous alternatives.
Walking down a drugstore aisle, most folks don’t pause to wonder what’s inside a lipstick or a lotion—let alone scan for names like Trimethyl Citrate. Yet, behind those barely-pronounceable chemicals, you’ll uncover the nuts and bolts of how many everyday products come together and stay safe. As someone with a background in science writing and an allergy-prone family, I’ve grown used to turning a critical eye to ingredient lists. Trimethyl Citrate, while not a household name, plays bigger roles in our routines than most realize.
Trimethyl Citrate usually turns up in cosmetics and skin care, working as a plasticizer. What does that mean in plain English? It makes things less brittle and more flexible. Take lipsticks: nobody wants a cracking, flaky product. By using this compound, manufacturers strike the balance between a firm stick and a smooth glide. Nail polish makers also turn to it, building strength into a lacquer without choking users on harsh solvents.
Sometimes it steps in as a scent fixative, helping perfume oils hang around longer on the skin. This role impacts everyone who enjoys a whiff of fragrance that doesn’t fade by noon. I remember testing perfumes at home and noticing which ones lasted longest—reading the label made it clear that certain stabilizers make all the difference.
Safety always ranks high for anything that touches skin or lips. Ingredients with a track record of irritation get the boot from dermatologists and from parents with sensitive-kid alarms like me. Trimethyl Citrate doesn’t ride the worry list for most oversight agencies. Europe’s Cosmetic Ingredient Review and the US Food and Drug Administration both list it as safe in the concentrations found in finished products. According to published research, the body processes and breaks down trimethyl citrate much like other citrus-based additives, which can matter for those trying to avoid accumulation of synthetic chemicals.
Natural and “green” beauty trends often push companies to find options with fewer environmental drawbacks or toxic byproducts. Trimethyl Citrate’s roots trace back to citric acid— that’s the same stuff in lemons and oranges— and methanol, both of which are widely studied. This heritage appeals to brands wanting to cut back on petroleum-based compounds, yet keep products reliable.
Product developers keep searching for better ways to balance stability, comfort, cost, and safety. Trimethyl Citrate does a solid job for now, but no single ingredient works perfectly in every formula. Sometimes it won’t mix well with all pigments or creams, which sends labs searching for the next breakthrough blend. In my time writing about product safety, I’ve seen companies field customer questions and adapt fast—especially as allergies and ingredient sensitivities become more visible. It pays to stay curious about what’s inside your bathroom cabinet. Reading up, raising questions, and paying attention to reactions makes a difference—especially for people with extra-sensitive skin or those looking for more sustainably-made options.
As more consumers dig into labels, the science shaping our daily routines will keep evolving. Trimethyl Citrate stands as one tool in a bigger kit for safer, longer-lasting personal care items—an ingredient you hardly notice, doing work you’d definitely miss if it disappeared.
Food additives often end up in headlines and daily conversations among anyone who keeps an eye on what goes into packaged goods. Trimethyl citrate, a compound made from the natural citric acid found in citrus fruits, serves most often as a plasticizer, but has also found its way into flavorings and pharmaceutical preparations. Its mild taste and ability to dissolve both water-based and oil-based ingredients invite food producers to rely on it in some candies, chewing gums, and supplements. People like me, who actually try to read labels before shopping, notice how many products carry ingredients whose names never show up in home kitchens.
It’s easy to get anxious over unrecognizable additives, especially with years of news about unexpected health effects from things that once seemed harmless. Trimethyl citrate has gone through industry scrutiny for decades, with regulatory agencies in Europe and the United States taking a hard look before letting manufacturers use it in food. In the United States, the FDA has declared it “Generally Recognized as Safe” (GRAS) for use in specified quantities, based on toxicology studies and research in both animals and people.
The European Food Safety Authority reviewed data and reached similar conclusions. Scientists studied its metabolism and found that the body breaks it down just as it does with citric acid from oranges or lemons. It gets converted, absorbed, and eliminated with no evidence of harmful buildup or byproducts. Regulatory science banks on decades of safety data, not just a few months of study. Still, regulators always stay open to new findings and research on health effects.
Most people don’t eat large amounts of trimethyl citrate every day. Exposure usually comes as a trace in processed sweets, some drinks, and supplements. At these low levels, studies report no negative health impact in healthy adults or children. Dietitians and toxicologists look not just at existing studies, but across related compounds and historic data to evaluate cumulative risk for the public.
That doesn’t mean everyone feels comfortable. Some consumers—like parents of kids with food allergies or health professionals treating patients with chemical sensitivities—demand clearer transparency from food brands. While trimethyl citrate allergies haven’t been documented, and the compound is not considered a top allergen, concerns about “chemical-sounding” ingredients never fully go away.
For people interested in health, following science-based recommendations trims away a lot of fear. Sticking to whole foods more often, reading ingredient lists carefully, and staying alert to new research helps form habits that don’t rely on the constant churn of food fads and scares. Someone battling chronic illnesses or already on a restricted diet could talk to their healthcare provider for more personal guidance.
A growing number of shoppers pick brands that make ingredient sourcing and additive choices public. Food companies could use their websites and product packaging to make their ingredient decisions and dosages clearer. Firms that run in-house tests or participate in academic partnerships tend to build trust more easily.
For folks like me, who try to balance healthy living and real life, long ingredient lists still mean double-checking, but not panic. By sticking close to solid science and demanding honest labeling, food shoppers can decide how comfortable they feel about additives like trimethyl citrate.
Trimethyl citrate lands in the spotlight mostly because of its role as a plasticizer. In the plastics world, not all materials stay soft and bendable on their own. Manufacturers add substances that loosen up rigid polymers, making packaging, toys, and cables more user-friendly. Trimethyl citrate, derived from citric acid, handles this job without carrying health or environmental baggage like phthalates do. In my own work with plastics, sourcing safer additives matters as clients push for non-toxic certifications. When children's products or medical items draw attention for chemical safety, using a biodegradable plasticizer cuts down on headaches and back-and-forth testing.
Not every plastic can go near food or skin. Regulators expect ingredients in forks, containers, and cosmetic bottles to meet high purity and low toxicity standards. Trimethyl citrate keeps up with these needs, showing up in films that wrap snacks or in tubes that hold lotions and creams. I've seen engineering teams swap in trimethyl citrate for traditional plasticizers in everything from lipstick cases to squeeze bottles after consumer groups raised concerns over exposure to questionable chemicals. The shift to safer compounds often starts with demands from global clients, trickling down through the supply chain until even small producers look for clean, approved alternatives.
In the pharmaceutical sector, not many additives can claim both technical function and safety for the patient. Trimethyl citrate shows up in coating materials for tablets, sometimes even in capsules that slow down and control medicine release. Picture a tylenol tablet that needs a smooth swallow—coatings with trimethyl citrate act as barriers but dissolve at the right place in your stomach. Some companies also take advantage of its solubility to help with taste-masking when a powder tastes bitter. In my experience, health agencies look for ingredients they can trust and that have a proven record in oral and injectable applications. Anything that assures fewer recalls or less regulatory scrutiny finds fans quickly with pharmaceutical clients.
Trimethyl citrate has become an alternative to phthalates, which lost trust over their environmental and health impact. Whether it’s a flexible PVC raincoat or a medical tube, companies no longer want to risk banned substances slipping through global compliance checks. Over the years, I've seen R&D labs convert product lines to citrate-based plasticizers for the peace of mind—they process well, don’t create off-odors, and support claims of “phthalate-free” or “non-toxic” products. End-customers notice these label changes, especially as parents and hospitals check the fine print.
Industries push for sustainability not only because of regulation but also because buyers care what goes into the products they use. Trimethyl citrate fits into circular economy goals since it comes from citric acid, commonly sourced from renewable feedstocks like corn or sugar. In conversations with supply teams, the question keeps coming up: “Can this ingredient help with compostable claims?” Trimethyl citrate often passes the test, supporting greener targets without sacrificing product quality. Using safer, plant-derived ingredients reduces exposure risks for factory workers and consumers alike. For any manufacturer facing tighter environmental rules, switching to this kind of compound matches both compliance demands and brand promises.
Trimethyl Citrate crops up in a lot of everyday items—think soft drinks, dessert toppings, personal care products, and even some medicines. Chemically, it’s an ester derived from citric acid and methanol, with uses that range from food additives to cosmetic stabilizers. Not every ingredient in a product is a household name, but scrutiny grows as more people wonder what these compounded names can do to health.
By and large, food and cosmetic industries consider Trimethyl Citrate safe in small amounts. Most folks who encounter it do so through processed foods or toiletries. The biggest worry comes with allergic reactions. Not everyone’s skin handles new additives, especially folks with eczema or fragrance sensitivities. A rash or a little redness may show up after using a lotion or cream that includes Trimethyl Citrate. The irritation doesn’t occur for everyone, but for those with skin sensitivities, new ingredients warrant a patch test before full application.
Oral exposure through food rarely leads to major side effects in healthy adults. A lot of the evidence points to it breaking down safely in the gut, eventually leaving the body through urine. Folks with citrate metabolism disorders, though, may feel the effects a bit stronger. These conditions are rare, but doctors recommend checking ingredient lists for anyone with a known metabolic problem. On the rare chance that a large dose is swallowed—think accidental ingestion in industrial settings—nausea or stomach discomfort could follow.
So far, Trimethyl Citrate doesn’t raise major alarms in the medical community, thanks to current research. The trouble is, studies focus mainly on immediate reactions and specific doses. There’s little data on what happens after years of low-level exposure through food and cosmetics. Since regulatory bodies like the FDA or European Food Safety Authority watch these ingredients closely, any patterns of harm would likely show up in wider consumer reports, but no ingredient gets a free pass forever.
Some simple habits go a long way here. If curious or concerned about an ingredient, check product labels. Cosmetic companies must list what’s inside, giving you a choice before purchase. People prone to allergies ought to perform a patch test on skin products with unfamiliar chemicals. Parents can lean on trusted pediatricians for advice, especially for infants and toddlers who may react unpredictably to new additives.
Research continues, as does oversight from consumer safety regulators. For those working in labs or factories, wearing gloves and masks limits accidental contact with concentrated forms. Consumer products contain much smaller amounts than raw materials, but following workplace safety rules stops mistakes before they start.
For most people, using items containing Trimethyl Citrate causes no harm. Anyone with special health conditions or unique skin sensitivities should check in with a healthcare provider to discuss any unusual symptoms. Ingredient transparency continues to improve, and reputable companies respond quickly to genuine concerns. Raising questions about what we put on our bodies and into our food leads to better awareness, smarter choices, and more targeted research into ingredient safety in the future.
Trimethyl citrate pops up in ingredient lists for everything from food to cosmetics. The word “citrate” often sounds natural—evoking citrus fruits and fruit-derived flavors. Still, the manufacturing process behind trimethyl citrate is more complicated than squeezing oranges or lemons.
This compound comes from citric acid. Citric acid itself occurs in nature—think of a tart lemon or a tangy orange. But in the real world, industrial citric acid almost always comes from a fermentation process using simple sugars and a mold called Aspergillus niger. The end result: a massive supply, powered by biotechnology rather than sun and soil. Trimethyl citrate comes next. Factories turn this citric acid into trimethyl citrate through a chemical reaction with methanol, called esterification. Workers and scientists fine-tune temperatures and conditions instead of waiting for nature to finish the work.
Labeling something “natural” stirs up a lot of trust from shoppers. I’ve met plenty of people who believe anything natural is better, purer, safer. Many companies play into this hope, emphasizing “natural” sourcing. With trimethyl citrate, though, most products on the shelf use the lab-created version, even if the starting point is a sugar grown in a field. Without direct extraction from a plant or fruit, calling it “natural” can be misleading. Regulations from government agencies, like the FDA or EFSA, focus on the origin and processing steps. They usually list trimethyl citrate as synthetic.
For people seeking clean-label food or sensitive to synthetic additives, it pays to know the difference. Not every synthetic is harmful, and not every natural compound offers safety. For trimethyl citrate, toxicity studies show a pretty good safety profile, so it’s not a “red flag” additive in scientific circles. In fact, many substances created in a lab can have higher purity than those extracted from nature, which sometimes include traces of allergens or contaminants.
Still, consumer trust relies on transparency. If someone picks up a lotion or a pack of flavored candies, no one wants to be tricked into thinking an ingredient comes straight from the harvest. As a parent, I look twice whenever I see scientific-sounding names in the pantry or bathroom. Trust builds from truth in advertising—for industry and for people at home.
The gap between “natural” and “synthetic” isn’t always clear from labels. A lot of confusion kicks in when marketing departments play up “citrus origins” or “nature-inspired” claims. Ingredient transparency would help. Using language backed by regulatory definitions should be standard practice. Offering information about source materials and processing methods builds trust and loyalty.
Clearer education about additives like trimethyl citrate matters for everyone who checks a label. People want to know what’s in their food, body wash, or medicine. For companies, building that relationship means going beyond the minimum legal requirements—offering online resources, clear Q&As, and customer service that doesn’t dance around questions of natural vs. synthetic.
References: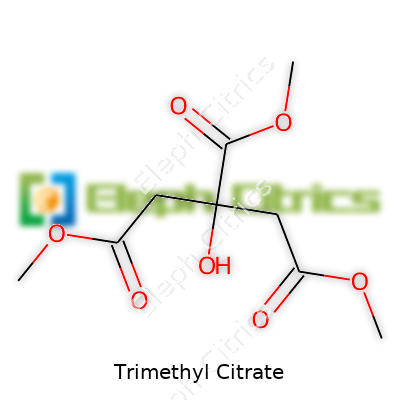
| Names | |
| Preferred IUPAC name | Trimethyl 2-hydroxypropane-1,2,3-tricarboxylate |
| Other names |
Citric acid, trimethyl ester Trimethyl 2-hydroxypropane-1,2,3-tricarboxylate Citric acid trimethyl ester Trimethyl 2-hydroxy-1,2,3-propanetricarboxylate Trimethyl ester of citric acid |
| Pronunciation | /traɪˈmɛθ.ɪl ˈsɪ.treɪt/ |
| Identifiers | |
| CAS Number | 77-93-0 |
| 3D model (JSmol) | `3D model (JSmol)` string for **Trimethyl Citrate** (PubChem CID: 6566): ``` C(C(=O)OC)C(CC(=O)OC)(C(=O)OC)O ``` |
| Beilstein Reference | 1721431 |
| ChEBI | CHEBI:86466 |
| ChEMBL | CHEMBL157596 |
| ChemSpider | 5279 |
| DrugBank | DB11152 |
| ECHA InfoCard | 03a5eaf6-6e49-4865-aa4b-c74fc6814a30 |
| EC Number | 205-786-4 |
| Gmelin Reference | 6137 |
| KEGG | C18691 |
| MeSH | D017728 |
| PubChem CID | 8774 |
| RTECS number | WY2625000 |
| UNII | 7Y76W8QM1V |
| UN number | UN3082 |
| CompTox Dashboard (EPA) | DTXSID501034254 |
| Properties | |
| Chemical formula | C9H14O7 |
| Molar mass | 290.29 g/mol |
| Appearance | Colorless transparent liquid or white crystalline powder |
| Odor | odorless |
| Density | 1.21 g/cm3 |
| Solubility in water | Soluble |
| log P | -0.07 |
| Vapor pressure | 0.015 mmHg (25 °C) |
| Acidity (pKa) | 7.6 |
| Basicity (pKb) | 8.72 |
| Magnetic susceptibility (χ) | -62.5×10⁻⁶ cm³/mol |
| Refractive index (nD) | 1.424 |
| Viscosity | 20 mPa·s (20 °C) |
| Dipole moment | 6.45 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 579.7 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1107.8 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -4045.7 kJ/mol |
| Pharmacology | |
| ATC code | A16AX16 |
| Hazards | |
| Main hazards | May cause respiratory irritation. Causes serious eye irritation. Causes skin irritation. |
| GHS labelling | GHS02, GHS07 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | H319: Causes serious eye irritation. |
| Precautionary statements | Precautionary statements: P261, P305+P351+P338 |
| NFPA 704 (fire diamond) | 1-1-0-NULL |
| Flash point | > 180°C |
| Autoignition temperature | 420 °C |
| Lethal dose or concentration | LD50 (Rat, oral): 6600 mg/kg |
| LD50 (median dose) | LD50 (median dose): Oral rat LD50 > 5000 mg/kg |
| NIOSH | WSS6285000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 0.3% |
| Related compounds | |
| Related compounds |
Citric acid Acetyltributylcitrate Acetyltriethylcitrate Triethyl citrate Tributyl citrate |