Trioctyl citrate entered the industrial scene as folks sought safer replacements for phthalate-based plasticizers. Before new regulations tightened the leash on older chemicals, manufacturers often leaned on phthalates, but scrutiny over toxicity and environmental impact shifted the market. Within this changing landscape, trioctyl citrate gained popularity, largely because it could offer flexibility for plastics while addressing rising safety demands. Research articles from the 1970s and 1980s drew attention to its lower volatility and smoother performance in flexible PVC materials. My own curiosity grew after seeing how European policymakers pushed for citrate alternatives across food packaging and toy manufacturing. This sort of transition never happens overnight; the adoption of trioctyl citrate was slow at first, but now, regulatory pressure and growing consumer awareness have made it a standard.
Trioctyl citrate serves as a plasticizer, giving otherwise brittle polymers the stretch and durability needed for everyday products. Whether in medical tubing, food wraps, adhesives, or nail polish, this compound proves reliable. I’ve run across it numerous times in technical documentation thanks to its oil-like consistency and lack of strong odor. Its transparency and low migration rates help it blend in. The market presents it in drums and totes, catering to industrial-scale users. Regulatory compliance sits front and center, with suppliers boasting about certifications showing food contact safety or meeting European pharmacopoeia standards.
This compound offers a clear, oily liquid state at room temperature. Trioctyl citrate has a molecular formula of C27H52O7, boiling between 225-255°C under reduced pressure. It weighs in at about 500 to 520 g/mol, features a refractive index of around 1.440, and tests show it holds a flash point above 225°C, which matters a lot in industrial settings that demand fire safety. Solubility in water falls at the low end, but it mixes well with various organic solvents. Unlike shorter-chain esters, trioctyl citrate resists sweating or exudation from finished plastic goods. These seemingly mundane stats can mean the difference between product recall and regulatory approval for manufacturers, highlighting why detailed property checks always play a role during formulation.
Quality control drives technical standards for this citrate ester. Purity usually exceeds 99%, Alkali content rarely surpasses 0.05%, and acid value comes in under 0.2 mg KOH/g. Manufacturers often provide certification certificates with detailed batch records, which suppliers use to reassure end-users. The labeling covers not just chemical identity but also recommended storage temperatures and handling methods. Many suppliers also include origin, shelf life (three to five years if properly sealed), lot number, and user safety notes. Modern traceability practices and digital catalogs make these details more accessible.
Production involves direct esterification of citric acid with n-octanol. This reaction takes place in an acid-catalyzed environment, often using sulfuric acid as the catalyst. Removing water produced by the reaction allows it to reach completion. My chemistry background makes me appreciate the precision required to keep impurities in check during this step. After the reaction, multiple washing and purification steps follow: neutralization, vacuum distillation, and filtration. The result is a pale, straw-colored liquid that matches rigid product specifications essential for downstream use.
Trioctyl citrate’s molecular backbone contains three octyl chains attached to a citrate core, which allows for some downstream modifications. In formulations, it doesn’t tend to react aggressively with other components, but its ester bonds can break under strong acidic or basic conditions, a consideration that matters in recycling or high-temperature processing. Derivatization sometimes enters the picture to introduce different alkyl groups for specialty plasticizer needs. In polymer blends, it influences compatibility, especially in polar matrices like PVC, without undue yellowing or tackiness. From a technical perspective, its performance stands out in applications where high resistance to leaching is required.
Chemists and suppliers use names such as TOC, citric acid trioctyl ester, or just trioctyl citrate. Some brands and catalogs may use specific trade names depending on their market. As regulations evolve, a quick search for these various terms has helped me cross-check safety guidelines or product restrictions across countries and industries.
Handling trioctyl citrate safely hinges on following established protocols. Direct skin contact should be avoided, and splash goggles with resistant gloves make sense on an industrial floor. The MSDS spells out low acute toxicity, but prolonged or repeated exposure has not been accorded a blank check, so proper ventilation remains a best practice. Storage in cool, dry warehouses away from oxidizers extends the product’s shelf life and reduces risk of destabilization. Transport guidelines classify it as a non-hazardous material under most shipping regulations, which often keeps logistics costs in check. Regulatory authorities in the European Union, United States, and China monitor allowable migration levels in food contact applications. End users must still maintain internal batch testing to ensure compliance, especially as new toxicology data comes in from ongoing research programs.
Trioctyl citrate features heavily in food contact plastics, such as wrapping films, bottle sealants, and flexible packaging. Medical-grade applications have found their stride in blood bags and tubing, where low toxicity and extraction levels meet tight safety requirements. I’ve seen its use spike in personal care products like cosmetics and nail polish removers, where odorless flexibility and skin safety tip the scales. The electronics industry taps into this ester in wire coatings, and building materials manufacturers roll it out in wall coverings and synthetic leather. With beverages, juices, and pharmaceutical coatings, the compound again checks the boxes for safety and performance. Shifting consumer demand for “phthalate-free” or “green” labels keeps pushing research teams to expand uses into biodegradable films and other novel packaging methods. Each new use case brings a fresh regulatory review, tying back to the need for rigorous supplier documentation.
R&D groups have pushed the limits on trioctyl citrate by testing its compatibility with emerging biopolymer blends and allergen-free packaging. In advanced studies, cross-linking techniques and nano-composite work aim to tune the migration rates and mechanical properties even further. Academic papers highlight experiments into improving recycling options by modifying the ester backbone. From my perspective, collaboration between chemical engineers and material scientists, especially under the hood of publicly funded projects, has fast-tracked new applications. Many companies have invested in pilot-scale projects to evaluate environmental footprints, hoping to showcase the benefits over traditional plasticizers and calm stakeholder nerves with numbers rather than marketing pitches.
Toxicity forms the backbone of every approval process. Toxicologists have tracked oral and dermal exposure routes in both animal models and limited human trials. Compared with phthalate esters, trioctyl citrate stands out with a higher median lethal dose and lower reproductive toxicity. Regulatory agencies flag only minor irritant risks under high-dose lab conditions, with negligible bioaccumulation in humans or wildlife, but continue to call for more longitudinal research. Some questions persist about the effects of chronic exposure, especially as microplastic research brings more scrutiny to materials that wind up in waterways or food chains. Independent groups in Japan and the European Union have invested considerable effort into tracing breakdown products to make sure no harmful metabolites escape notice.
The market momentum behind trioctyl citrate looks set to increase, as regulations lean away from questionable phthalates and toward proven-safe plasticizers. Packaging giants and medical technology leaders both recognize the compound’s potential to sustain and support new biodegradable solutions, opening doors for further environmental certifications and wider public acceptance. Research pipelines bring together high-performance blends, advanced composites, and sustainable feedstock approaches, which could shift trioctyl citrate’s supply chain over the next decade. As I keep reading the fine print on consumer product packaging and medical device inserts, it’s clear that trioctyl citrate has carved out a spot as a go-to solution for safer, flexible plastics. The next era will revolve around making its lifecycle greener and its applications even broader, with regulatory science and consumer trust always in the spotlight.
Trioctyl citrate does not show up in everyday headlines, but its presence can be traced inside items many of us handle every single day. Think of a kid’s chewy candy from the corner store, or a phone charger that stays flexible even in winter. This colorless liquid, known for keeping things pliable and easy to use, works quietly behind the scenes.
Trioctyl citrate often finds a place in plastics, especially those found in packaging, medical equipment, and even toys. Manufacturers use it to soften polyvinyl chloride (PVC). If you ever noticed how some cables stay flexible in your toolbox and don’t crack in the cold, there’s a good chance a plasticizer like trioctyl citrate got blended in. Food contact materials—like cling wraps and some bottle coatings—also pull from a similar playbook, using this ingredient to help prevent brittleness.
Food is another unexpected area. Food scientists reach for trioctyl citrate as an additive, known by the number E1505. Chewing gum and processed cheese often rely on it. The compound keeps gum chewy and cheese spreadable, sidestepping crumbling and drying out. It passes safety checks in measured doses and doesn’t stick around in the body for long.
People get nervous seeing additives with complex names, and I’ve had parents in my neighborhood ask whether these chemicals are safe. Trioctyl citrate meets strict standards in both the United States and Europe. Food safety agencies, including the FDA and EFSA, call it safe within limits. Studies show it’s not toxic at regular amounts found in foods or plastics. Still, any plasticizer in contact with food must get heavy scrutiny, as small kids and people with sensitivities need extra protection.
My experience with community environmental groups taught me parents worry more about “what if” rather than what’s typical. They want to know the limits exist and are enforced. Facts matter: Regular safety reviews do happen, drawing on lab tests and real-world monitoring.
Debate over plasticizers usually follows concern about phthalates, many of which have been restricted due to health risks. Companies searching for replacements often try trioctyl citrate. It doesn’t share the worst of those health risks. Still, researchers watch for emerging science that might flag new issues, especially as we put more artificial materials in contact with our food.
Waste is another issue. Plastics stick around in the environment long after use, and even safe ingredients like trioctyl citrate often travel with them. More research focuses on making sure these chemicals don’t slip into water or soil in harmful ways.
In the search for safer, greener softeners, some companies test biodegradable alternatives, while others cut back on plastics. Transparent labeling matters. People want a say in what goes near their kids’ food or ends up in their home. Regulations need to reflect both up-to-date science and what communities care about most. Certified safer chemicals and clear information make life easier for everyone, letting us balance convenience with long-term well-being.
Plastics touch food every day—cling wrap, container linings, squeeze bottles. Manufacturers use a range of additives to get just the right flexibility or strength. One of these ingredients is trioctyl citrate, known by some as TOC or tri-n-octyl citrate. It pops up in food packaging because it works well as a plasticizer, especially for PVC. But as a consumer, every additive deserves a closer look.
Trioctyl citrate helps make plastics soft and bendy. Companies pick it for its stable, non-volatile nature. That just means TOC doesn't break down or release gases under normal storage conditions. Unlike older phthalates, which have raised more than a few health concerns over the years, TOC doesn’t seem to build up in our bodies. It has a reputation for being low in toxicity.
Researchers have poked and prodded TOC in animal studies. They’ve looked for allergic reactions, reproductive issues, and long-term harm. So far, data show it passes most toxicology hurdles. The European Food Safety Authority reviewed it and found no sign of carcinogenic, mutagenic, or reproductive problems at normal exposure levels. The U.S. Food and Drug Administration allows TOC use in vinyl plastics that touch food, as long as concentrations stay within set limits.
Safe, though, doesn’t mean risk-free. Plastics and their additives can migrate—small amounts move from the packaging into the food. Scientists have tested how much TOC ends up in food with daily use. Results point to migration values far lower than official safety thresholds. The watchdog agencies built in large safety margins to protect people of all ages, from infants chewing on bottle nipples to adults eating takeout from food trays.
Still, most folks outside science labs don’t want a chemistry test with their lunch. Stories of plasticizers like DEHP and DINP causing trouble have made shoppers jumpy about anything added to packaging. Families, schools, and anyone with food allergies want clear answers, not just reassurance. Transparency makes a real difference—not just in technical data but in how manufacturers talk to the public.
If a product uses TOC, labels rarely spell out what that means or what studies back up its safety. Grocery shoppers have to dig deep or trust government bodies to review the research. The presence of alternatives—some of them plant-based plasticizers—suggests there's room for innovation and choice.
Better safety testing keeps moving the target. Scientists now explore how mixtures of chemicals interact, not just single ingredients alone. Advocacy groups push for packaging built from fewer, simpler inputs. I’ve found that consumers feel more empowered when they can track where their food packaging comes from and what it contains. Supermarkets and brands can earn loyalty if they go beyond the bare minimum required by law and share details proactively.
Trioctyl citrate stands out as one of the safer plasticizers in modern packaging, based on current data. That doesn’t mean it should coast on its record. As testing gets more sophisticated, and as pressure grows for cleaner, more transparent supply chains, companies can take the lead by supporting rigorous science and keeping communication open for everyone who eats or drinks anything packaged in plastic.
Trioctyl Citrate catches the eye for its clarity, thickness, and the feeling of almost effortless spread when getting mixed into a solution. It pours as a clear, oily liquid and holds no scent, which often matters in products needing to avoid strong odors. At room temperature, the liquid refuses to freeze into a solid block, only thickening as things cool down. Its boiling point sits high—over 400°C—so it hardly ever vaporizes during standard processing or use, keeping risks of loss and fire low.
This compound won’t mix with water but blends easily with oils and many organic solvents. That difference in behavior means it works well in places where you want to keep water out or create a lasting, flexible finish—think vinyl flooring, rubber coatings, and endless plastic parts. The low water solubility lets it stay in formulas longer without leaching out, especially in humid conditions where other additives might bleed or sweat off.
Trioctyl Citrate isn’t easy to break down. It shrugs off mild acids and bases, and heat rarely phases it unless you hit seriously high temperatures. This stability lowers maintenance costs and boosts product lifespans, and reduces headaches for those responsible for quality control in manufacturing.
Chemically, it’s known as an ester—specifically, the product of citric acid and octanol. This structure gives it flexibility as a plasticizer. It softens materials without turning them sticky or tacky once cured, which sets it apart from alternatives like phthalates, long debated for health reasons.
In my experience working with product safety, having a plasticizer that doesn’t trigger regulatory headaches brings real value. Trioctyl Citrate has landed in everything from children’s toys to food-contact materials because it’s far less likely than many older plasticizers to cause hormonal disruption or other long-term health concerns. The European Food Safety Authority (EFSA) reviewed it and ruled it holds low toxicity when used carefully. But as with any chemical, it’s smart to handle it with gloves in large quantities to avoid skin irritation.
Factories using Trioctyl Citrate need good air circulation, since high concentrations could irritate the lungs over long exposure. I’ve seen setups where poor ventilation led to worker complaints—not from toxicity, but from sheer discomfort as vapors built up. Automation and sealed systems help keep this under control, reducing risk for everyone in the workplace.
Plastic manufacturers have leaned into Trioctyl Citrate, especially after big public pushback against phthalates in everything that touches food or kids. It softens polymers just enough, without breaking them down or changing their appearance, and sticks around for the long haul in finished goods. On the environmental side, it decomposes faster than many phthalates, lowering its footprint after disposal—but full biodegradation still takes more than a standard landfill cycle.
Researchers keep experimenting to lower production costs and boost green processing. Moving toward raw materials made from renewable resources could pave the way for a safer and cleaner lifecycle. Trioctyl Citrate already leaves a lighter mark, but tighter controls and smarter chemistry promise even better options soon.
Trioctyl Citrate shows up in all sorts of industries, from plastics to personal care. At its core, this liquid plasticizer keeps flexible products bendy and stable for the long haul. Even with its reputation for low toxicity, that doesn’t mean you can treat it like harmless water. Basic respect in storage and handling protects your health and keeps mishaps at bay.
Too many compounds get ruined or get risky just because folks stash them in the wrong spot. For Trioctyl Citrate, heat and open air are the nemeses. Exposure to hot or damp conditions speeds up its breakdown, and things get sticky — literally and legally, if you ship or use subpar stuff. A dry, cool place keeps the liquid clean and consistent.
I’ve worked in a warehouse where skipping basic precautions cost us a lot of product after just one power failure. People sometimes ignore the advice and hope for the best, but temperature swings warp chemicals. You end up dealing with leaks, odd smells, and suppliers questioning your quality.
Heavy-duty drums and tightly sealed containers are the only way to go. I don’t mess around with old or rusty barrels, since metals corrode and leave traces that can mess with downstream processes. Quality polyethylene or stainless steel drums work well — anything else and you risk contamination or a bad spill once you try to move it.
Always label containers clearly. Mixing mistakes or accidental exposure are a headache in the best-case scenario, and in the worst case, someone gets hurt. I've seen teams fumble through a mess simply because someone forgot to update a label, and the stress is never worth it.
Every workplace I’ve ever been in has its own culture about personal protective equipment. Some folks pull on gloves and goggles out of pure habit, others play it casual. With liquids like this, gloves and splash-proof goggles are non-negotiable. No one wants a skin rash or to rinse their eyes at the emergency wash station, trust me.
Spills happen even with the best planning. Keep oil-absorbent pads and proper drainage nearby, especially in busy transfer areas. Training new team members on how to sweep up and dispose of even small leaks pays off long-term. It saves cleaning costs and builds trust between safety supervisors and everyone else.
Ventilation is another point I always look for. Even chemicals categorized as “low toxic” can irritate lungs or trigger allergies in some folks if fumes build up. Good airflow in storage and transfer spaces matters.
Shipping chemicals like Trioctyl Citrate crosses into regulatory territory fast. Rules keep changing and authorities don’t cut slack. From my experience, proper paperwork and compliant packaging stop most headaches at border checks. Hazmat teams at logistics firms want to see that nothing’s leaking, and that all seals and signs are in place. No shortcuts. I’ve seen full shipments get stuck for weeks over a missing inspection stamp, which wrecks delivery timelines and cash flow.
Truth is, safety keeps people loyal and sharp. Clear protocols, solid team training, and the right gear — these steps anchor a safer environment. If you put the right systems in place, you not only avoid fines and product loss but also show respect for everyone clocking in each day. That mindset spreads, and soon enough, keeping Trioctyl Citrate secure and stable is second nature, not a bureaucratic box to tick.
Trioctyl citrate pops up in plenty of products, from plastic toys to food packaging, thanks to its reputation for adding flexibility without piling on the worries that come with heavy metals. Some call it “green” because it comes from citric acid and octanol, which both have roots in natural processes. But turning those raw materials into trioctyl citrate takes chemical tricks only seen in big factories, and the end result feels far from something squeezed out of a lemon.
Labeling a chemical as biodegradable grabs attention. Companies want products that won’t linger in landfills or drift unchanged into rivers. Tests show trioctyl citrate breaks down faster than older phthalates. In lab setups, bacteria eventually chew away at it, transforming it into smaller pieces that nature can handle. Looking at real-world waste systems, it does decompose over weeks or a few months. Compare that to the years or even decades some plasticizers stick around, and trioctyl citrate looks less burdensome.
Still, the story’s not black and white. Trioctyl citrate’s speed of breakdown depends on what else sits around it. Cold temperatures, certain soils, and low-microbe environments slow things to a crawl. The claim only really holds up under lab conditions or compost piles packed with the right bacteria. In landfills, air and microbial action run low, so the process stretches out. That means bottles or wrappers using the chemical could take a lot longer to fade away than a compost test would suggest.
Even if trioctyl citrate breaks down eventually, there’s more to ask. Its production chain stretches from industrial farms producing citric acid to chemical plants running on fossil fuels. Every step stacks up resource use, energy burn, and pollution. Once used in plastics, trioctyl citrate seeps into soils or water. Studies so far don’t link it to major harms in wildlife at common doses. Fish and crustaceans usually shake it off in small amounts, and humans face low risk at today's exposure levels.
Some synthetic additives linger because we build them to last and resist water. Trioctyl citrate sits closer to the middle—less stubborn than many, but not as fleeting as those in nature's own recycling bin. Microplastics and chemical leachates still pose problems, and even “safer” chemicals play a role in building up broader pollution.
Switching from old-school phthalates to trioctyl citrate helps keep some toxics out of food wraps and playgrounds. Yet it doesn’t mean a full step into a world where plastics and additives work in lockstep with nature. Folks pushing for cleaner alternatives often ask for plant-based plasticizers that break down quickly in any backyard garden, not just special lab setups. Some European regulators urge manufacturers to back up “biodegradable” labels with clear, independent field tests, so buyers know what they’re getting.
As someone who walks past overflowing dumpsters and rivers flecked with plastic bits, I notice how easy it is to feel better about a chemical swap without looking deeper. Trioctyl citrate serves as a reminder: progress tends to come slow, and real environmental gains call for following the story from factory to final fate. People and companies willing to ask tough questions before leaping into new materials help shift more than labels—they help deliver change you can see.
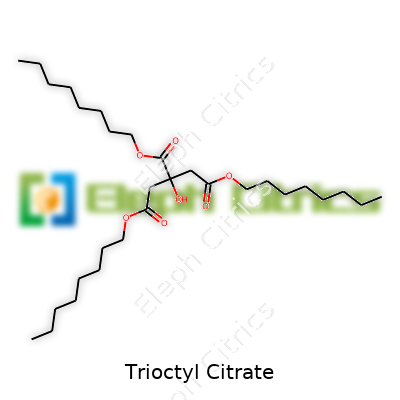

| Names | |
| Preferred IUPAC name | Tris(2-ethylhexyl) benzene-1,2,3-tricarboxylate |
| Other names |
CITRIC ACID TRIOCTYL ESTER Trioctyl 2-hydroxypropane-1,2,3-tricarboxylate Octyl Citrate n-octyl citrate Trioctyl 2-hydroxy-1,2,3-propanetricarboxylate TOTM Trioctylcitrat Citroflex 8 Octyl Citrate Ester |
| Pronunciation | /traɪˈɒk.tɪl ˈsɪ.treɪt/ |
| Identifiers | |
| CAS Number | “78-42-2” |
| 3D model (JSmol) | `3D model (JSmol)` string for **Trioctyl Citrate**: ``` CCCCCCCCOC(=O)C(CCOC(=O)CCOCCCCCCCC)(COCCCCCCCC)OC(=O)CCOCCCCCCCC ``` |
| Beilstein Reference | 1461112 |
| ChEBI | CHEBI:53327 |
| ChEMBL | CHEMBL2170547 |
| ChemSpider | 12313 |
| DrugBank | DB11274 |
| ECHA InfoCard | ECHA InfoCard: 100.048.366 |
| EC Number | 205-066-5 |
| Gmelin Reference | 1533918 |
| KEGG | C18677 |
| MeSH | D013987 |
| PubChem CID | 6566 |
| RTECS number | TSCAQ7000000 |
| UNII | G9WDU5A60Y |
| UN number | UN3077 |
| CompTox Dashboard (EPA) | DJ5R8I01EU |
| Properties | |
| Chemical formula | C₃₀H₅₂O₈ |
| Molar mass | 546.78 g/mol |
| Appearance | Colorless or pale yellow oily liquid |
| Odor | Odorless |
| Density | 0.983 g/cm3 |
| Solubility in water | Insoluble |
| log P | 8.31 |
| Vapor pressure | < 0.01 mm Hg (20 °C) |
| Acidity (pKa) | 4.7 |
| Basicity (pKb) | 6.86 |
| Magnetic susceptibility (χ) | Diamagnetic |
| Refractive index (nD) | 1.444 |
| Viscosity | 350 cP (25°C) |
| Dipole moment | 1.78 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 751.85 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1487.6 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -10500 kJ/mol |
| Pharmacology | |
| ATC code | A10BX12 |
| Hazards | |
| GHS labelling | GHS07 |
| Pictograms | GHS07, GHS09 |
| Signal word | No signal word |
| Hazard statements | May cause an allergic skin reaction. |
| Precautionary statements | Precautionary statements: P261, P262, P280, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-1-0 |
| Flash point | 210°C |
| Autoignition temperature | 210°C |
| Lethal dose or concentration | LD50 (oral, rat): >5000 mg/kg |
| LD50 (median dose) | > 2900 mg/kg (rat, oral) |
| NIOSH | NA3325000 |
| PEL (Permissible) | No PEL established. |
| REL (Recommended) | 2-8°C |
| IDLH (Immediate danger) | Unknown |
| Related compounds | |
| Related compounds |
Acetyl tributyl citrate Tributyl citrate Trimethyl citrate |