Looking back at the history of tripotassium citrate monohydrate, this compound comes out of efforts to transform naturally occurring acids into more practical forms for both food preservation and health management. In the late nineteenth and early twentieth centuries, advances in industrial chemistry led to the commercial manufacturing of citrate salts, including sodium and potassium varieties. As food preservation started relying more on chemical means, potassium salts gained attention since customers wanted lower sodium alternatives for cardiovascular and kidney health. The monohydrate form developed from a need to control energy and moisture during production, with manufacturers learning that one water molecule helps stabilize the salt without compromising its performance.
Tripotassium citrate monohydrate, often just called potassium citrate in the industry, appears as a white, granular, and odorless powder. It slips easily between the fingers, dissolves quickly in water, and leaves only a faintly salty taste. Its main job lies in regulating acidity, acting as a buffering agent, and supplying potassium. You’ll see it in countless sectors: health supplements, sports drinks, medical nutrition, processed foods, even some technical or laboratory uses. Food technologists find it valuable for its pH control, while doctors and nutritionists recommend it for managing certain kidney stones or to restore electrolyte balance. On every shelf, from pharmaceuticals to bakeries, it’s more common than most realize.
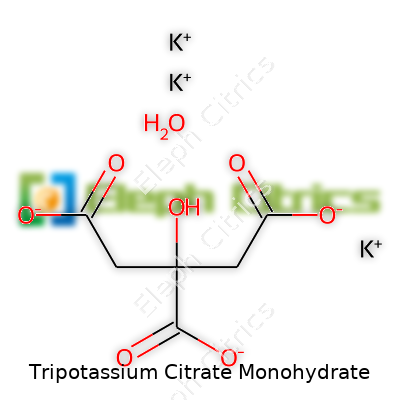
This compound packs a molar mass of about 324.42 g/mol and a formula of K3C6H5O7·H2O. It stands up to heat, remaining stable below 230°C, and absorbs moisture straight from open air—one reason extra care goes into storage and transport. As for solubility, throw a few grams into any glass of water and it vanishes, which is essential for any food or beverage application needing a uniform solution. It won’t ignite easily and doesn’t support combustion, which suits high-temperature processing environments. Chemically, the water of hydration plays its part in how well it flows and mixes in industrial setups.
Manufacturers have to stick close to international standards—think FCC, USP, or E number E332(iii). Purity typically needs to sit at 99% or above, with heavy metals and other contaminants under tight control to pass regulatory checks. Labels must show not only the chemical name and grade but also a batch number, manufacturer, country of origin, and storage conditions, offering transparency all the way from the distributor to the end user. These technical details become non-negotiable if the compound is bound for medical or dietary uses, as patients might rely on it for critical care.
Most industrial batches start with a potassium salt—often potassium carbonate or potassium bicarbonate—and citric acid in highly controlled conditions. Heat and water help the two get along, finalizing in a crystallization step where temperature and evaporation give just the right monohydrate form. What stands out is the precision the process demands: any slip in proportions or temperature can lead to an undesired hydrate or an off-white color, making the whole batch unusable for some applications. Modern setups catch impurities at every step, using filtration and recrystallization to ensure a pure result every time.
Chemists and product engineers have spent years mapping how the compound interacts with acids, bases, and other salts. It takes only a mild acid to convert tripotassium citrate to other citrate salts or release citric acid, giving food scientists options for tweaking taste or nutritional profiles. Blend it in solutions with calcium or magnesium and it sometimes forms insoluble precipitates, which affects both food texture and supplement bioavailability. Through careful chemical manipulation, new forms and blends keep appearing to meet different wants—whether improved dissolving rates or compatibility with new drug formulations.
Tripotassium citrate monohydrate carries several names depending on the country or industry. Some refer to it simply as “potassium citrate,” but others prefer to clarify with “triple potassium salt of citric acid,” “E332(iii),” or “potassium citrate monohydrate.” Pharmacies sometimes abbreviate it with K-citrate. Despite the variety of names, the core substance stays the same, giving stability to global supply chains that need consistent quality for health and food applications alike.
Trusted organizations like the Food and Drug Administration, European Food Safety Authority, and World Health Organization have all hammered out rigorous safety standards for tripotassium citrate monohydrate. Handlers need gloves and masks when dealing with large quantities, as dust could cause irritation over time. In workplaces, spills should get cleaned up promptly, with airflow kept high to avoid unnecessary inhalation. For consumers, the main risks lie in over-consumption; too much potassium might trigger hyperkalemia, a known danger for people with kidney problems. Proper labeling and staff training offer real defense buffers against unintentional misuse.
Doctors often reach for potassium citrate in the fight against kidney stones and acidosis, especially for patients who struggle with sodium or need to boost potassium safely. Nutrition supplement firms blend it into electrolyte powders, vitamins, and “recovery” beverages for athletes. Food producers value its role in processed cheese, baked goods, and jams, where it helps create both stability and the right mouthfeel. Winemakers and brewers use it for acid adjustment, getting the right profile without harsh flavors. As plant-based diets gain ground, non-dairy cheese makers also rely on this salt for texture and shape, since it’s free of animal byproducts and hypoallergenic.
Research teams dig deeper every year, exploring tripotassium citrate’s ability to support cardiovascular health, bone strength, and metabolic function. Investigators track how it modulates blood pressure, interacts with other nutrients in the gut, and whether it could replace sodium-based additives in prepared foods. Engineers in the pharmaceutical sector keep eyeing delivery systems that can modulate potassium release—for instance, developing slow-release pills or powder blends that don’t clump or degrade over time. These efforts anchor tripotassium citrate firmly within the next generation of both foods and medicines.
Scientists have scrutinized potassium citrate for decades, given how potassium touches everything from muscle contraction to heart rhythm. Most reports show it’s safe within approved use levels, but repeated studies reinforce calls for caution among those with impaired kidneys or who take certain medications. The main challenge for toxicologists is distinguishing the effects of the potassium ion versus the citrate ion—each having its own profile. Ongoing studies now focus on long-term exposure, potential for cumulative toxicity, and whether genetically influenced metabolisms handle supplements differently. Transparent data monitoring and clear public health advice help people safely handle and consume products containing it.
Tripotassium citrate’s role keeps expanding across sectors aiming to reduce sodium, support kidney health, or deliver key minerals in palatable ways. As food regulation tightens, demand for additives that double as nutrients grows—a space where this compound excels. Biodegradable packaging and technical products might soon include citrate derivatives as “green” additives to speed composting or stabilize biodegradable plastics. Medical researchers look at custom blends and slow-release forms tailored for chronic health issues. Smart factories now use real-time quality monitoring to spot deviations and cut down waste, all while delivering a product that meets new sustainability targets. There’s growing momentum for further research into plant-based products, where safe and effective potassium sources line up with consumer values and regulatory trends alike.
Tripotassium citrate monohydrate grabs a lot of attention in the medical world, and the reasons make sense. Kidney stones can feel like sharp rocks scraping their way through the urinary tract—painful and all-too-common. Doctors often turn to potassium citrate to help keep kidney stones from coming back. By making urine less acidic, tripotassium citrate lowers the odds of crystals forming. Folks with gout or metabolic acidosis, where the blood gets too acidic, sometimes get prescribed this salt as well. Years of research back this up. The American Urological Association recommends citrate therapy for those at risk of recurrent stones.
Beyond its stone-fighting powers, this compound supports heart health. Low potassium can trigger heart palpitations, weakness, or muscle cramps. Potassium citrate fills that gap. The balance matters because our nerves, muscles, and heart rely on the right amount of potassium to fire signals and contract smoothly. I’ve seen people on certain diuretics develop low potassium, feeling washed out and weak, only to bounce back with supplements. Doctors often favor citrate salts over simple potassium chloride, especially for folks who need to tame blood acidity or prevent stones.
Walk down any grocery store aisle, and this ingredient shows up more often than most realize. Manufacturers use it as a buffering agent, which means it keeps products from turning too acidic. Soft drinks, powdered drinks, and dairy-based desserts benefit from it, avoiding sharp sour tastes and prolonging shelf life. Makers of processed cheese use potassium citrate to keep textures smooth—no strange oily separation or weird dryness. I’m picky about cheese sauces, and the difference is easy to spot by taste and texture.
Why not just use sodium salts to handle this job? High sodium links to high blood pressure and health problems over time. Potassium citrate gives makers a way around this. By switching to potassium instead of sodium, food keeps its flavor, and those of us battling hypertension or watching salt intake get a break. The Centers for Disease Control and Prevention backs up the need to lower sodium in processed foods, given sky-high rates of high blood pressure in the United States. As consumers pay closer attention to nutrition labels, ingredients like tripotassium citrate grab a larger role.
It’s easy to forget the demands in industry. In detergents, potassium-based salts help lift stains and keep machines from building up limescale. I grew up washing dishes by hand and later worked with folks tracking equipment wear in dishwashers and industrial kitchens. Adding potassium compounds to cleaners stretches out the working life of pipes and pumps. Water treatments also feature potassium citrate, especially in places where water supplies carry heavy hardness or minerals. Less scale means lower costs in repair and replacing parts.
Potassium citrate’s “greener” profile lines up with current efforts to clean up supply chains. Unlike older additives that rely on phosphorus or more hazardous chemicals, tripotassium citrate does not feed algae blooms in lakes or rivers. Municipalities looking to reduce water pollution look for this kind of ingredient. Investing in updated water softening and cleaning products makes a difference at a bigger scale—something I’ve watched as local governments adjust contracts for cleaning and maintenance supplies.
Tripotassium citrate monohydrate may sound like one of those technical chemicals best left behind lab doors, but anyone who’s worked in a pharmacy, food factory, or laboratory knows the fuss over storage isn’t just ticking boxes. The stuff breaks down in overdamp cupboards, clumps up when humidity sneaks in, and picks up other flavors and odors from shared cabinets. Mishandling isn’t just a small nuisance—it directly threatens the safety and effectiveness of every batch.
The biggest thing on my mind, whenever I see those white crystals, is humidity. Moisture grabs onto that monohydrate fast, and those neat grains quickly turn sticky or lumpy. Ideally, tripotassium citrate monohydrate belongs somewhere well below 60% relative humidity. Desiccant packs in airtight containers have saved plenty of shipments from disaster in the storeroom. Even in controlled environments like pharmaceutical plants, I’ve watched how stockrooms rely on digital hygrometers and strict policies for checking the seals on everything.
A stable temperature ties right into keeping the powder’s quality intact. Nobody wants to haul in bags from a sweltering warehouse or one that freezes over in winter. I make sure to keep storage areas between 15°C and 25°C—a range most everyday storerooms can manage. Sudden spikes or drops prompt more frequent quality checks. Temperature swings can speed up water loss or even changes in crystal structure, making the material unreliable.
Tripotassium citrate monohydrate loves to soak up what’s around it—not just water, but also stray dust, spilled liquids, or even strong-smelling chemicals. Dedicated shelves and bins can make a world of difference. In practice, that means using food-grade or pharmaceutical-grade containers, keeping lids tight, wiping up any surrounding spills fast, and storing well away from anything strong-smelling or reactive. No one wants odd odors drifting over into food or medicine production.
Direct sunlight or harsh lighting can trigger chemical changes that degrade tripotassium citrate monohydrate. My go-to is always a cool, shaded corner, well away from window ledges. Even overhead lights shouldn’t beam directly onto open bins or bags. Opaque or tinted containers help here, and more than once, I’ve rescued mislabeled shipments by moving them out of the light in time.
Clarity on every container keeps mistakes at bay. Expiry dates, batch numbers, and hazard labels should stand out, no matter how rushed the day gets. In my experience, clear, big print on every lid and side avoids confusion and keeps teams from reaching for the wrong product. I always recommend a last-in, first-out rotation, so nothing sits around long enough to degrade.
The right conditions come down to dry air, steady room temperature, sealed containers, good labels, and separation from contaminants or strong odors. A little extra attention goes a long way. Accidents, wasted product, and failed test results force everyone to slow down. Attention to detail in storage, learned from hands-on experience, makes a difference right down the line—from manufacturing to final use.
Automated climate controls and better moisture indicators on packaging are helping. Updating staff on storage protocols and running regular training is essential so that every team member—whether a new hire or a 20-year veteran—treats tripotassium citrate monohydrate with the care it deserves.
Tripotassium citrate monohydrate turns up on the ingredient lists of drinks, processed foods, and even some medications. After hearing the chemical name, instincts kick in to question what it actually does to the body. Tripotassium citrate itself shows up as a potassium salt. Food producers like it for its ability to regulate acidity, improve shelf life, and beef up potassium content. People use it in the medical world as well, especially for kidney stones or certain urinary tract issues.
The body actually thrives on potassium. Doctors always mention how potassium keeps the heart, muscles, and nerves running smoothly. Tripotassium citrate breaks down in the gut, delivering a healthy dose of potassium and helping reduce acid in urine. The body handles it just as well as potassium from a banana, given you don’t go overboard. One research paper published by the U.S. National Library of Medicine points out that moderate potassium intake keeps blood pressure in check and helps keep kidney stones from returning. Too much potassium, though, can be tough on those with kidney problems.
Global regulatory agencies, including the U.S. Food and Drug Administration (FDA) and the European Food Safety Authority (EFSA), have both signed off on tripotassium citrate when used properly. The FDA places it on the "generally recognized as safe" (GRAS) list for food additives. EFSA reviewed its uses and found regular consumption from food or medicine stays far under any harmful level for healthy folks.
That said, the same research highlights why doctors caution those with kidney issues or who take certain heart medications. If the kidneys struggle to remove extra potassium, levels can build up and spell trouble—muscle weakness, abnormal heart rhythms, and even heart failure. Experts recommend monitoring potassium levels for anyone who already walks a tightrope with their electrolyte balance.
Most people taking in tripotassium citrate through food and the occasional supplement won't run into any real risk. I remember double-checking the back of a sports drink during marathon training. The potassium comes in handy after long races, since dehydration and hours on the road will drain electrolytes. Doctors sometimes hand out prescription versions for those with kidney stone issues. The key is that these folks get regular monitoring and know exactly how much to use.
People with normal kidney function almost never run into trouble, according to studies published in peer-reviewed science journals like the American Journal of Kidney Diseases. Problems creep in if someone loads up on supplements or mixes in drugs such as ACE inhibitors that mess with potassium regulation. Folks with kidney disease, Addison’s disease, or who are taking certain blood pressure meds simply need to follow medical advice before consuming extra potassium.
One practical approach includes reading food labels, asking questions at the pharmacy, and talking to healthcare professionals before adding any supplement to the routine. No one food or ingredient makes a diet perfect or poisonous—context always matters. Tripotassium citrate monohydrate plays a useful role for most people without health concerns. As with anything, balance stays essential, and honest conversation with a healthcare provider proves far more helpful than internet scare stories or guesses.
Tripotassium citrate monohydrate shows up in clinics and pharmacies for one main reason—helping keep certain minerals balanced in the body. It gets a lot of attention for its ability to slow down the formation of kidney stones and to support people with issues like renal tubular acidosis. If you have ever tangled with kidney stones, you know that the last thing anyone wants is a repeat bout of that pain. My own experience in working alongside chronic kidney stone patients made that clear: getting the dose right means fewer sleepless nights and hospital visits.
Doctors usually start adults on a dose around 10 to 20 grams per day, split into two or three doses after meals. Most specialists aim for just enough to keep the urine potassium level normal and the urine pH between 6 and 7.5. Children get much smaller amounts, often tailored to body weight. To put numbers behind that, you see recommendations landing anywhere between 1 to 2 mEq of potassium per kilogram of body weight each day for pediatric patients.
Fact is, personal health history carves out the dose. Someone with healthy kidneys can usually clear extra potassium without much trouble, but folks with kidney problems need a more careful approach. Too much potassium, and you could wind up with risky heart rhythms.
Doctors lean heavily on lab tests before and during treatment. People differ widely—diet, age, other medical conditions, and regular medicines all tweak how much is right for each person. Many of the patients I saw had to check in regularly for blood potassium and urine pH. Failing to check these numbers risks either not doing enough or, worse, causing serious side effects.
Tripotassium citrate monohydrate works because it breaks down into potassium and citrate inside the body. Citrate binds to calcium, which keeps stones from forming. The recommended dose targets the sweet spot where you help the bladder and kidneys without tipping into problems.
Too low a dose doesn’t prevent stone formation. Too high a dose, and you could trigger stomach upset, nausea, or dangerous levels of potassium in your blood. High potassium throws off muscle and heart function. Medical literature points to reported cases of muscle weakness and dangerous arrhythmias linked to poor dosing decisions. The FDA also flags the importance of close monitoring, especially for patients with cardiovascular or kidney disease.
Doctors rely on clear patient communication, regular blood work, and updating the health plan as life changes. If you have new meds or catch a virus, your body might not handle potassium like it did before. Patients need straight talk on warning signs—tingling, confusion, or an irregular heartbeat means pick up the phone and check in.
Following the right dosage comes from listening: to the body, to lab results, and to the doctor’s guidance. Too many people with kidney stone history skip doses or adjust them on their own, which rarely ends well. Keeping everyone on the same page, with good follow-through and education, makes the biggest difference in staying out of trouble.
Medical guidelines from the National Kidney Foundation and research published in clinical nephrology journals all suggest similar dosage ranges, with a strong emphasis on seeing the full picture—age, other medical issues, and lab results guide every adjustment. Doctors prescribing tripotassium citrate rely on these studies, using evidence to keep patients safe and pain-free.
Tripotassium citrate monohydrate pops up on the label of a number of medications, especially those prescribed to manage kidney stones or keep urine less acidic. Getting too much or taking it the wrong way though—it brings a list of side effects that can’t be ignored. I’ve known a few too many patients who thought it was as safe as a multivitamin, but the story runs deeper.
Most people expect a pill to work quietly in the background. With tripotassium citrate, your stomach may protest early on. Nausea is a regular visitor; some folks talk about cramping, bloating, or a burning feeling beneath the ribs. Those who aren’t expecting it get a real wakeup call. Food sometimes helps cushion the blow, but not always. I’ve seen cases where people stop taking it, convinced that it can’t be worth the discomfort.
Bathroom visits can get more complicated too. Diarrhea or loose stools crop up in some, while others end up with the opposite problem. In rare cases, vomiting causes enough concern to send people back to the clinic.
The kidneys usually keep potassium in line, but potassium from supplements can push blood levels too high. I’ve worked with patients who needed potassium, but the risk of “hyperkalemia” (too much potassium in the blood) means regular lab tests matter a lot. That’s where things can turn serious—muscle weakness, slowed heartbeat, and dangerous heart rhythms.
Elderly adults and folks with kidney trouble walk a much tighter rope. Their bodies can’t clear extra potassium efficiently. If a supplement like this interacts with other medicines—blood pressure drugs, water pills, or non-steroidal painkillers—the odds of side effects rise further.
A handful feel dizzy or get a metal taste for days. Though few people connect muscle twitches or tingling skin to potassium supplements, neurologists and pharmacists see these links often enough to flag them for safety.
It’s easy to grab something from the pharmacy, but the real lesson lies in checking why it’s used and weighing out personal risk. Doctors usually order blood tests before suggesting any potassium product. That single move has saved more patients from cardiac complications than anything else. Staying hydrated and spacing out the dose during the day also helps lower the chance of stomach issues.
For those who already take medications for chronic disease—blood pressure pills or anything that affects the kidneys—it pays to talk with a healthcare provider about risk. Keeping a list of current medicines at appointments helps spot risky combinations before trouble sets in. Over-the-counter drugs like ibuprofen can quietly bump up the risk, even if the package says nothing about potassium.
Eating more fruits and vegetables rich in potassium offers a steadier option for some. For my patients who can’t safely handle more potassium or don’t like the side effects, other approaches to preventing kidney stones—hydration, lower salt diets—carry less risk. The best result often comes from teamwork: the prescriber, the pharmacist, and the patient staying in the loop, watching both side effects and benefits closely. No supplement replaces a real conversation about risk and safety, and the way forward often relies on paying attention to what the body says along the way.
| Names | |
| Preferred IUPAC name | potassium 2-hydroxypropane-1,2,3-tricarboxylate monohydrate |
| Other names |
Potassium citrate monohydrate Tripotassium 2-hydroxypropane-1,2,3-tricarboxylate monohydrate Potassium citrate hydrate Citrate of potash monohydrate Monohydrate tripotassium citrate |
| Pronunciation | /traɪ-pəˈtæsiəm saɪˈtreɪt ˌmɒnəˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 6100-05-6 |
| Beilstein Reference | 15612770 |
| ChEBI | CHEBI:31645 |
| ChEMBL | CHEMBL1200985 |
| ChemSpider | 21476477 |
| DrugBank | DB14537 |
| ECHA InfoCard | 07a76446-ebfe-4d00-b303-c6bfe6e1f1a9 |
| EC Number | 209-891-3 |
| Gmelin Reference | 1043 |
| KEGG | C00703 |
| MeSH | D007354 |
| PubChem CID | 167604 |
| RTECS number | TS8050000 |
| UNII | L0BN1IC10Y |
| UN number | UN1760 |
| CompTox Dashboard (EPA) | DTXSID4020293 |
| Properties | |
| Chemical formula | K3C6H5O7·H2O |
| Molar mass | 324.41 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.98 g/cm³ |
| Solubility in water | Very soluble in water |
| log P | -3.3 |
| Vapor pressure | Negligible |
| Acidity (pKa) | 8.6 |
| Basicity (pKb) | 8.5 |
| Magnetic susceptibility (χ) | -85.0e-6 cm³/mol |
| Dipole moment | 2.03 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 365.2 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -2094 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -374 kJ/mol |
| Pharmacology | |
| ATC code | A12BA03 |
| Hazards | |
| Main hazards | May cause mild skin and eye irritation. |
| GHS labelling | GHS07, GHS Hazard Statement: H319 |
| Pictograms | GHS07 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| NFPA 704 (fire diamond) | Health: 1, Flammability: 0, Instability: 0, Special: - |
| Lethal dose or concentration | LD50 (oral, rat): 5400 mg/kg |
| LD50 (median dose) | > 5400 mg/kg (Rat, oral) |
| NIOSH | KM2850000 |
| PEL (Permissible) | Not established |
| REL (Recommended) | 30 mg/kg bw |
| Related compounds | |
| Related compounds |
Monopotassium Citrate Dipotassium Citrate Potassium Citrate Sodium Citrate Citric Acid Calcium Citrate Magnesium Citrate |