Over the years, the journey of trisodium citrate dihydrate tells a story shaped by science, industry, and everyday needs. Scientists first drew attention to its potential in the 18th and 19th centuries, exploring its basic buffering and flavoring powers. It soon moved from the lab bench to more practical uses in food preservation and stability because soda ash and citric acid production ramped up with industrialization. Big names in chemistry, from Carl Wilhelm Scheele to Jöns Jacob Berzelius, played roles in uncovering how this salt acts as both a tool for balance and a foundation for further discovery. By the time global trade sharpened in the 20th century, nearly every country with chemical or food processing capability embraced trisodium citrate as both a staple and a signal of modern safety standards.
Trisodium citrate dihydrate keeps showing up in places as common as lemonade stands and as technical as advanced medical labs. Its official label points to a formula, Na3C6H5O7·2H2O, and a clean, almost invisible presence—no color, slight tartness, and a quick reaction with anything acidic. Its roots in the food industry took off because it not only tastes mild but also pulls double duty as a stabilizer and preservative. Many food processors and beverage companies rely on it to tweak flavor balance, stop spoilage, and keep products shelf-stable. Pharmacies and hospitals stock it for use as an anticoagulant and pH regulator in solutions, and veterinary medicine calls on it for similar purposes. Trisodium citrate dihydrate slips into detergents and cleaners too, where it softens water and boosts cleaning power.
Handling trisodium citrate dihydrate means working with a powder that dissolves quickly and completely in water, letting chemists and food scientists measure or mix with pinpoint precision. The salt holds onto two water molecules, which gives it just the right amount of moisture for stable storage but not enough to trigger clumping or caking under regular indoor conditions. At room temperature, it almost refuses to pick up more moisture from the air, so it flows easily from bag to beaker. Chemical tests show it as mildly alkaline, with the solution hovering above neutral on the pH scale. This mild alkalinity supports its use in buffering formulations, holding a product steady even if acids or bases sneak in. While strong acids will break it down and release citric acid, in daily use it stays reliable—no strange smells or colors, no tricky side-reactions.
Quality standards for trisodium citrate dihydrate come directly from global pharmacopeias and food safety codes. Check the sack or drum, and you’ll see clear declarations for purity—often 99% or higher—and tight limits on contaminants like heavy metals, arsenic, and fluoride. Each shipment includes a certificate of analysis listing granular size, drying loss, pH in solution, and pass-fail marks on microbial loads. Food-grade labels come with detailed batch numbers for traceability, lot expiry dates, and handling recommendations in plain terms for anyone who needs to scoop, blend, or dissolve the material. Some suppliers color-code packaging to separate grades: pharmaceutical, laboratory, and technical. This attention to transparency feeds right into modern audit and recall processes, which depend on clear records and verifiable test results.
Factories make trisodium citrate dihydrate by mixing citric acid, usually sourced from fermented corn or cane sugar, with a sodium carbonate or bicarbonate solution. The two react, giving off carbon dioxide bubbles and leaving behind a rich, complex mix right at the sweet spot of neutralization. Operators carefully control pH to make sure the reaction tilts fully toward the trisodium salt, not any of the partially neutralized cousins. After settling and filtering, the solution cools to coax crystals out of the liquid. These crystals get washed and dried under conditions that lock in two water molecules for every formula unit, keeping the salt flowable and pure. Because every variable from pH to temperature alters crystal size and clarity, real experience in the plant makes a difference between a flaky, hard-to-handle powder and the silky, easily dissolved salt that buyers want.
Trisodium citrate dihydrate plays a flexible role in both simple mixtures and more reactive systems. It takes on acids gently, shifting the pH and lending stability to foods and medications. Mixed with calcium salts, it keeps them from precipitating out, which matters in everything from cheese making to blood storage. Chemists often use it as a precursor for citric acid recovery or as a chelating agent, trapping metal ions to prevent unwanted side reactions. Heating trisodium citrate above 150°C can drive off water, and under more forceful thermal treatment, it breaks down into citraconic and aconitic acids—rarely useful for most industries but a fascinating sidetrack for those studying organic chemistry. Some researchers modify the salt to create slow-release forms for gradual nutrient delivery in soil or to experiment with encapsulation for tailored drug release profiles.
Depending on the catalog or industry, trisodium citrate dihydrate goes by a handful of names that all get at its sodium-citric acid core. Food labels often spell it out as sodium citrate or E331, while chemistry references prefer trisodium citrate dihydrate or 2-hydrate. Older texts might list it as citric acid sodium salt, and outside North America, workers sometimes use terms like trinatriumcitrat. Specialty suppliers sell it under branded product names, each with minor tweaks for granulation, dryness, or packaging but all tracing back to the same chemical structure.
Working with trisodium citrate dihydrate usually presents low risk, with recognized low toxicity and minimal environmental impact when handled by the book. Operators wear gloves and masks to avoid skin irritation or breathing dust, and spills get swept up with standard cleanup kits. Food and pharmaceutical-grade supplies must pass through tightly monitored hygiene routines to prevent cross-contamination. Facilities follow strictly posted storage and handling guidelines: cool, dry, and protected from anything acidic that could trigger unwanted reactions. Regulatory updates push manufacturers and users to document every shipment, maintain clean batch records, and keep safety data sheets within easy reach for quick response in case of exposure. Waste streams containing trisodium citrate dihydrate run through standard wastewater treatments, breaking down naturally into safe byproducts, echoing its low environmental footprint.
Trisodium citrate dihydrate found a home in more industries than most people realize. Food and beverage companies rely on it for tartness, salt regulation, and as a safeguarding buffer against microbial growth. Dairy plants use it to keep cheeses smooth and slice-friendly. Pharmacists compound it into oral rehydration solutions, anticoagulants for blood transfusions, and treatments for metabolic acidosis. Cleaning product companies turn to its chelating power to soften water and boost detergent effects without harsh phosphates. Textile and paper factories see it as a stabilizer that improves dye uptake and helps in waste treatment. Even oil-field operations started using sodium citrate blends to prevent scaling and boost mud performance, showing that a salt pulled from citric acid can live many industrial lives. In my own kitchen, I’ve used food-grade trisodium citrate to nail the smoothest cheese sauces and to keep lemonade bright even after a few days in the fridge.
Universities and R&D labs keep searching for new tricks from trisodium citrate dihydrate. Teams investigate better forms of encapsulation for pharmaceutical use, aiming to deliver medication more efficiently. Designers of advanced cleaning agents test blends that push cleaning power up without adding harsh chemicals. New discoveries in water treatment suggest it can bind to novel contaminants and break down emerging pollution types, making it a favorite of researchers looking for environmental solutions. Some projects explore its role in controlling heavy metals in soil and water, an area that could become more important as regulations tighten.
Extensive research confirms that trisodium citrate dihydrate stands among the safest of food additives. Animal studies and long-term toxicity tracking give it a green light for even high daily intakes, provided total sodium consumption stays within dietary limits. Workers handling bulk quantities face low hazard profiles, mainly related to dust exposure rather than chemical toxicity. Regulatory reviews by agencies like the European Food Safety Authority and the U.S. Food and Drug Administration rank it as a safe addition for nearly every human application. Ongoing monitoring ensures new scientific insights are folded back into safety standards, and open literature checks for any rare adverse reactions or unexpected environmental impacts. Despite all these reassurances, companies keep looking for improvements in purity and updated testing to keep risks as close to zero as possible.
Looking forward, trisodium citrate dihydrate’s future stretches out in many directions. Demand in food processing and beverage production still rises year after year, especially as global nutrition shifts call for more stable, fortified products with clear ingredient lists. Its strength as a cleaner, safer chelating agent puts it firmly in the conversation for green chemistry and sustainability efforts, presenting a proven way to lift performance without adding new risks. Biomedical scientists target it for even more clever uses in controlled-release technology and smart packaging that keeps supplies safe longer. While the basic recipe for making trisodium citrate probably never changes, tweaking particle size, water content, and purity standards could unlock new niches and meet tougher regulatory targets. In the end, its combination of practical safety, reliable performance, and open possibilities make it a salt that keeps adapting as needs and technology evolve.
Walk through any decent-sized grocery store and you’ll find boxed mac and cheese, canned soups, sodas, and jelly candy. Look at the label, and there’s a good chance you’ll see “trisodium citrate dihydrate.” It keeps foods tasting sharp but not sour, smooth but not oily. My years in foodservice showed me how this ingredient keeps cheese sauce creamy, stops crystals from taking over frozen desserts, and lets that tangy bite shine in lemony drinks.
At its core, this white crystalline compound comes from citric acid—a natural substance found in citrus fruits. Add sodium, and chemistry gives us trisodium citrate dihydrate. For food makers, it pulls several important levers. It balances acidity, controls pH, and helps flavors do their thing without overwhelming the palate. Cheese for nachos or pizza flows so smoothly because this powder keeps the fats and proteins from drifting apart. In sodas or sports drinks, it softens harsh edges without drowning out the zing customers crave.
Hospitals also put this stuff to work. Trisodium citrate dihydrate shows up in IV bags, drawn for its power to keep blood from clotting before tests or transfusions. As an anticoagulant, it binds calcium ions that blood cells rely on to stick together. That means lab results stay accurate, and donated units of blood won’t clog up equipment. Medical teams favor it because it’s safer than some older chemicals, causing fewer side effects in patients.
Beyond food and medicine, trisodium citrate dihydrate finds a spot in detergents and cleaners. Its knack for grabbing metal ions (like magnesium and calcium in tap water) keeps soap from turning into ugly scum. I’ve seen this firsthand using hard well water at home—dish soap didn’t work nearly as well until we tried a brand with citrates on the label. Dishes turned out cleaner with less rinsing.
For ordinary users, safety grabs a lot of attention. Regulatory groups like the FDA and EFSA mark trisodium citrate as safe at normal levels in food and beverages. Problems tend to show up only with huge doses—more than anyone gets from a balanced diet. In medical settings, nurses and doctors track and control dosage tightly. For home cooks and eaters worried about “chemical-sounding” names, it’s worth remembering that this ingredient builds its clean record on decades of careful study.
With all these uses, it makes sense to stay aware of how much processed food goes into a weekly menu. Cooking at home with basic ingredients keeps salt and additives like trisodium citrate dihydrate in check. At the same time, demanding honest labeling from manufacturers gives everyone more power to decide what’s on their plate.
Trisodium citrate dihydrate sounds more complex than the simple jobs it does every day: keeping foods fresh, drinks crisp, medicines sterile, and soaps effective. Modern life relies on small helpers like this. As always, awareness and a little label reading are the best tools for anyone who wants both convenience and control.
Food labels sometimes look like chemistry textbooks. Trisodium citrate dihydrate pops up fairly often, usually in beverages, processed cheese, or powdered drinks. It's basically the sodium salt of citric acid, the chemical that gives lemons and limes their tartness. It looks like a white, powdery substance but dissolves quickly in water, which makes it pretty handy in manufacturing food and drink.
In the kitchen, and in the factory, trisodium citrate acts like a buffer. It keeps acid levels in check, stopping things from turning too sour or too bland. It helps cheese melt smoothly, so it’s often found in slices and spreads. In sports or electrolyte drinks, it helps control the acidity, so your mouth doesn’t pucker after every sip.
It’s natural to wonder if eating something with a chemical name is a good idea. The United States Food and Drug Administration lists trisodium citrate dihydrate as "generally recognized as safe" for most people when used in normal food amounts. That means plenty of scientists and reviewers have checked the effects on humans and animals.
The European Food Safety Authority agrees, setting a daily intake limit that’s quite high compared to what a typical person would consume. Most people wouldn’t hit that limit unless they’re eating huge amounts of processed foods.
The most common worries involve sodium intake. Trisodium citrate does contain sodium, and most of us eat more of that than we should, mainly from salt. Extra sodium isn't great news for blood pressure. I had to watch my own sodium when a routine blood test showed my levels pushing the upper range, so I started eyeing ingredient lists more carefully. For anyone watching their salt, it’s wise to keep an eye on processed foods, as that’s often where sodium sneaks in.
Some people are sensitive to citrates. I’ve met a few folks with acid reflux who find that foods high in citric acid or its salts can trigger discomfort. That's not dangerous for everyone, but worth considering if you notice a pattern with these foods.
Both the FDA and major health organizations allow the use of trisodium citrate dihydrate in food at levels typical for manufacturing. There’s no evidence showing it causes cancer or toxicity at these amounts. For most healthy adults and kids, eating food seasoned or preserved with trisodium citrate comes with far more benefit than risk, particularly when compared to salts or other regulators that can pose greater risks.
Reading food labels makes a difference if you want to manage your sodium. Compare a homemade meal to a boxed mac and cheese: the homemade dish often wins on less sodium and fewer additives. If processed food is a regular part of your diet, consider varied options. Simple swaps like snacking on unsalted nuts or fresh fruits help bring down your intake.
The science shows that trisodium citrate dihydrate is safe in the amounts found in food. For most people, it doesn’t pose a risk, especially as part of a balanced diet. Eating a mix of whole and packaged foods works for most families. It’s about balance and awareness—tools just as valuable as any ingredient in your kitchen.
Trisodium citrate dihydrate looks like a simple white powder at first glance, but storing it the right way really shapes its effectiveness and safety. I remember the first university lab I joined—our store of common reagents felt neglected, and every once in a while, someone would grab a lumpy bag that had clearly absorbed some moisture. Trisodium citrate is hygroscopic, which means it loves to suck up water from the air. If it sits out, humidity turns it from a free-flowing powder to a solid brick. This sounds minor, yet it can ruin measured dosing for everything from buffer creation in biochemistry to food processing batches.
Experience taught me to keep this material sealed up tight every time. Storage works best in a cool, dry place—think room temperature, away from direct sunlight, and out of range from sinks or steam lines. Anything warmer or exposed to moisture can clump the powder and trigger early breakdown. Keep the powder in a tightly closed container, ideally a bottle or jar made from glass or high-grade plastic. Those commercial containers with screw caps or snap locks give solid protection.
A big part of E-E-A-T in science comes from knowing the way materials act in real-world settings. Inconsistent storage habits not only change the shelf life, but also risk introducing impurities to reaction mixes. In food production, off-spec powder brings in unexpected flavors or poor product texture. Buffer solutions in a lab environment suffer when water-laden citrate stops dissolving like it should. Not to mention, any attempt at accurate weighing gets thrown off once the powder cakes up. These effects can waste time and add costs at any production scale.
Good labeling goes hand in hand with smart storage. It’s easy to forget when a powder gets opened, so marking the date on the container helps catch problems early. Keeping work areas tidy limits cross-contamination. I learned this the hard way; once, an unlabeled container led to a mix-up between food-grade and technical-grade citrate. Food safety relies on knowing exactly what’s in the container and how it was handled. This lesson stuck with me: don’t cut corners, even with seemingly simple compounds.
Solid habits matter more than expensive gear. Use desiccant packs—like silica gel pouches—inside storage cabinets. These absorb stray moisture and keep the powder loose. For bigger batches, sealed buckets with rubber gaskets do the trick, especially in humid climates. Train everyone handling the material on why sealing and storage make a difference, not just how. The best results always come from treating even everyday materials with care, following consistent procedures, and staying alert to changes in appearance or flow.
Trisodium citrate dihydrate serves many roles, but its usefulness depends on how it’s stored. Dry, clean, sealed containers in controlled environments stop headaches before they begin. Keeping up these standards isn’t about overkill—it’s a habit built on experience and respect for the material, making workflows smoother and outcomes more reliable across the board.
Picture a big tub of processed cheese or a bottle of clear lemon-lime soda. Behind every smooth texture and every fizzy sip, someone in a lab decided whether to use trisodium citrate dihydrate or the anhydrous form of this sodium salt. At first glance, it's easy to wonder why scientists care about water content in a substance that’s basically "the same thing." From my time consulting for ingredient suppliers, I saw what a difference that watery detail can actually make, from food production to pharmaceutical mixing.
Trisodium citrate dihydrate carries two water molecules locked into its structure, while the anhydrous version skips the water. That little change looks pretty technical, but it throws off the math in a recipe or a drug formulation. Every time factories swap one for the other, they run into a difference in actual sodium and citrate delivered by weight. Add dihydrate to a batch where anhydrous was specified, and you might fall short of the required ingredient strength since part of your measurement is just water.
I’ve watched production lines freeze up over a batch gone wrong because someone missed this detail and aimed for weight instead of pure substance. One study from the Journal of Food Science highlighted problems with flavor consistency when water content slipped in unmeasured. Dosing in pharmaceuticals can behave the same way, with dosing errors possible if water content doesn't get factored into the calculation.
Dihydrate forms softer, slightly clumpy crystals that tend to attract moisture from the air. In a humid facility, that means more caked-up powder and less shelf stability. Anhydrous trisodium citrate stays dry longer and handles rough shipping better, showing up as powdery and free-flowing, which suits automated production lines. I’ve tested both in snack seasoning factories, and the difference in storage and handling jumps out fast. The dihydrate will start clinging to scoops, clogging up dispensers during a muggy summer.
Large-scale food processors often lean on the anhydrous form simply for machine reliability and control. Candy makers like the steady flow and predictable blending. Companies cranking out sports drinks and sodas get more leeway in picking dihydrate, especially with climate-controlled storage. In drug manufacturing, a pharmacist friend pointed out that anhydrous forms reduce the risk of unwanted chemical reactions tied to moisture, improving final stability for critical tablets.
In chemistry labs, particularly those I visited in biotech startups, the solubility of trisodium citrate can make or break a process. The anhydrous form dissolves faster thanks to the lack of extra water bonds, making it handy for anything high-speed or requiring quick clean-up. Dihydrate can lag behind in these settings, sometimes causing uneven mixing. In bottled drinks, the difference seems minor, but in time-sensitive processes, that speed can spare companies downtime and waste.
Every formulation project I’ve worked on circles back to one rule: do the math and check the label. Manufacturers and R&D chemists should train teams to recognize which form is listed and adjust weights to match the actual citrate and sodium needs. This prevents over- or under-dosing, keeps flavors on target, and meets safety standards. Audits and cross-checks help, especially as more ingredients arrive from global suppliers with different conventions.
Making a choice between trisodium citrate dihydrate and anhydrous comes down to how the product is made, stored, and used. Fact-checking every shipment and recipe batch keeps surprises off the line, ensures safety in medical dosing, and delivers what customers expect. Small chemical differences end up shaping everything from snack taste to the stability of pills. No detail is too small.
Trisodium citrate dihydrate pops up everywhere—food, medicine, cleaning agents. Behind its long name hides a simple goal: regulate acidity, keep products stable, and sometimes even prevent kidney stones. It does this job well if stored the right way. Even a chemical like this, which seems rock-solid, faces limits. Shelf life isn’t just a suggested timeline; it’s about keeping guarantees on safety and performance.
Manufacturers usually report a shelf life of three to five years for trisodium citrate dihydrate. This isn’t just a guess—it’s built on lab testing and real-world storage trials. A cool, dry environment, away from sunlight, stops clumping and chemical changes. Temperature and humidity speed up breakdown. What I’ve seen on industrial shelves matches what the science says: left open or in humid spaces, the salt starts to turn lumpy, might pick up musty odors, or display yellow discoloration. Properly sealed and away from moisture, it stays usable well past the marked date, but nobody wants to risk product recalls or medical failures by testing those limits.
Shelf life isn’t “just paperwork.” In the food world, expired or degraded trisodium citrate can mess with flavor, mess with food safety, or kill the desired texture of a product. Imagine a soft drink at a deli counter: bad acidity control means lost customers. Pharmaceuticals don’t offer any room for error. Medication batches require perfect purity, and a slip in chemical stability can land a whole run in the trash or even cause harm if it slips through.
I’ve had colleagues in manufacturing talk about long-forgotten bags that started clumping—their assumption was that sealed bags would guarantee freshness. Humidity and bad storage can undo all those best practices. In one facility, a faulty HVAC unit ramped up air moisture and cost thousands in raw material and finished goods. Nobody wants to waste money like that, especially not on something as basic as a storage mistake.
Cool, dry, sealed—those are the main rules. Polyethylene bags inside sturdy drums work best for big stockpiles. Out in the open air, only what’s needed for daily use should get exposed. Anyone working in a bakery, lab, or processing plant knows this rule: moisture is the enemy. Hygroscopic materials like trisodium citrate suck up water from the air, so one careless day with a lid open can start a chain reaction of wasted ingredients and ruined products.
Beyond best practice labeling, tracking batch numbers and rotation dates makes it easier to catch potential problems before they reach the customer. Modern inventory software helps, but a good old-fashioned checklist on the warehouse wall doesn’t hurt.
Getting shelf life right means looking beyond shipping labels. Better training keeps workers alert to sneaky moisture sources and accidental cross-contamination. Packaging engineers continue to look for new solutions, like advanced liners or desiccants that add extra layers of protection. For anyone at home: airtight containers, kept cool and dry, will almost always stretch useable life. If something smells off or looks odd, safe disposal beats risk.
Nobody wins when shelf life gets ignored. In a world that counts on reliable food, medicine, and safe cleaning products, honoring the true shelf life of trisodium citrate dihydrate pays off.
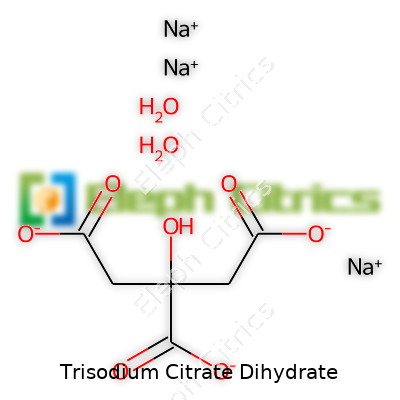
| Names | |
| Preferred IUPAC name | Trisodium 2-hydroxypropane-1,2,3-tricarboxylate dihydrate |
| Other names |
Sodium citrate Citrosodine Trisodium 2-hydroxypropane-1,2,3-tricarboxylate dihydrate C6H5Na3O7·2H2O E331 Sodium citrate dihydrate |
| Pronunciation | /traɪˈsəʊdiəm ˈsɪtreɪt daɪˈhaɪdreɪt/ |
| Identifiers | |
| CAS Number | 6132-04-3 |
| Beilstein Reference | 3591818 |
| ChEBI | CHEBI:61377 |
| ChEMBL | CHEMBL1201472 |
| ChemSpider | 5013 |
| DrugBank | DB09122 |
| ECHA InfoCard | ECHA InfoCard: 03-2119441184-37-0000 |
| EC Number | 200-675-3 |
| Gmelin Reference | 3874 |
| KEGG | C00711 |
| MeSH | Dihydrates; Citrates; Sodium Compounds |
| PubChem CID | 6224 |
| RTECS number | GE8300000 |
| UNII | 1U8M74KX7D |
| UN number | UN3077 |
| Properties | |
| Chemical formula | Na₃C₆H₅O₇·2H₂O |
| Molar mass | 294.10 g/mol |
| Appearance | White crystalline powder |
| Odor | Odorless |
| Density | 1.76 g/cm³ |
| Solubility in water | Very soluble |
| log P | -3.3 |
| Vapor pressure | <0.01 hPa (20 °C) |
| Acidity (pKa) | 7.7 |
| Basicity (pKb) | 7.7 |
| Magnetic susceptibility (χ) | `-48.0 × 10^-6 cm^3/mol` |
| Refractive index (nD) | 1.396 |
| Viscosity | Viscous liquid |
| Dipole moment | 7.2 D |
| Thermochemistry | |
| Std molar entropy (S⦵298) | 254.0 J·mol⁻¹·K⁻¹ |
| Std enthalpy of formation (ΔfH⦵298) | -1567.2 kJ/mol |
| Std enthalpy of combustion (ΔcH⦵298) | -2391.2 kJ/mol |
| Pharmacology | |
| ATC code | B05CX04 |
| Hazards | |
| Main hazards | May cause eye, skin, and respiratory tract irritation. |
| GHS labelling | GHS07, Exclamation mark, Warning, May cause irritation to eyes and skin |
| Pictograms | GHS07,GHS09 |
| Signal word | Warning |
| Hazard statements | Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008. |
| Precautionary statements | P264, P270, P305+P351+P338, P337+P313 |
| NFPA 704 (fire diamond) | 1-0-0 |
| Flash point | > 210 °C |
| Autoignition temperature | 210°C (410°F) |
| Lethal dose or concentration | LD50 (oral, rat): 5400 mg/kg |
| LD50 (median dose) | 5400 mg/kg (rat, oral) |
| NIOSH | WFN5CV1276 |
| PEL (Permissible) | PEL not established |
| REL (Recommended) | 30 mg/kg body weight |
| IDLH (Immediate danger) | Not listed |
| Related compounds | |
| Related compounds |
Citric acid Monosodium citrate Disodium citrate Trisodium citrate anhydrous |